Efanesoctocog Alfa Population Pharmacokinetics and Repeated Time-To-Event Analysis of Bleeds in Adults, Adolescents, and Children with Severe Hemophilia A
- PMID: 40123211
- PMCID: PMC12202194
- DOI: 10.1002/jcph.70008
Efanesoctocog Alfa Population Pharmacokinetics and Repeated Time-To-Event Analysis of Bleeds in Adults, Adolescents, and Children with Severe Hemophilia A
Abstract
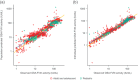
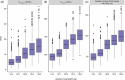
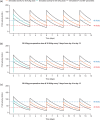
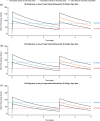
Efanesoctocog alfa is a first-in-class high-sustained factor VIII (HSF) replacement therapy for treatment of hemophilia A. This article presents population pharmacokinetics (PopPK) of efanesoctocog alfa and repeated time-to-event (RTTE) analysis of bleeding episodes in adults/adolescents (≥12 years of age) and children (<12 years). The final PopPK dataset contained pooled data from 277 patients (4405 post-dose factor VIII [FVIII] activity records) from two Phase 1/2a studies (NCT03205163; EudraCT 2018-001535-51), and three Phase 3 studies, XTEND-1 (NCT04161495), XTEND-Kids (NCT04759131), and XTEND-ed (NCT04644575). The PopPK model developed was a linear one-compartment model including body weight effect on clearance and volume of central compartment; Asian race was identified as a statistically significant covariate on clearance. The final PopPK model adequately described the FVIII activity-time profiles in adults, adolescents, and children with once-weekly (QW) efanesoctocog alfa 50 IU/kg, consistent with experience in XTEND-1 and XTEND-Kids. Bleeding episodes in participants in XTEND-1 and XTEND-Kids were characterized by an RTTE model with a Weibull base hazard and effect of FVIII activity modeled by a power effect. The RTTE model showed the probability of being bleed-free in 1 year with efanesoctocog alfa 50 IU/kg QW regimen was >70% across all age groups, consistent with the observed clinical outcomes in the Phase 3 trials of highly effective protection from bleeding episodes in patients with severe hemophilia A, which validates the model's prediction of the long-term bleed hazard.
Keywords: adults; efanesoctocog alfa; hemophilia A; pediatric; population pharmacokinetics; time‐to‐event analyses.
© 2025 Sanofi and Pharmetheus AB. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
Nancy Wong, Marek Demissie, Annemieke Willemze, Sreeraj Macha, and Craig Benson are employees of Sanofi and may hold shares and/or stock options in the company; Pratik Bhagunde and Suresh Katragadda were employees of Sanofi at the time of this study; Joakim Nyberg was a paid consultant to Sanofi (through Pharmetheus) during this work.
Figures
References
-
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(S6):1‐158. - PubMed
-
- Berntorp E, Hermans C, Solms A, Poulsen L, Mancuso ME. Optimising prophylaxis in haemophilia A: The ups and downs of treatment. Blood Rev. 2021;50100852. - PubMed
-
- Collins PW, Obaji SG, Roberts H, Gorsani D, Rayment R. Clinical phenotype of severe and moderate haemophilia: Who should receive prophylaxis and what is the target trough level? Haemophilia. 2021;27(2):192‐198. - PubMed
-
- Malec L, Matino D. Targeting higher factor VIII levels for prophylaxis in haemophilia A: a narrative review. Haemophilia. 2023;29(6):1419‐1429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

