Relevance of anti-platelet factor 4/heparin antibodies and platelet activation in systemic inflammatory diseases and thrombosis disorders: insight from the COVID-19 pandemic
- PMID: 40123654
- PMCID: PMC11929090
- DOI: 10.1016/j.rpth.2025.102701
Relevance of anti-platelet factor 4/heparin antibodies and platelet activation in systemic inflammatory diseases and thrombosis disorders: insight from the COVID-19 pandemic
Abstract
Background: The increased interest in anti-platelet factor 4 (PF4)-heparin complex (anti-PF4/H) antibodies following the COVID-19 pandemic has established them as crucial players in immunothrombosis.
Objectives: We aimed to investigate the involvement of anti-PF4/H antibodies during COVID-19 and after vaccination, particularly in patients with systemic inflammatory disease (SID).
Methods: This retrospective study analyzed the presence of anti-PF4/H antibodies and their ability to induce platelet activation in COVID-19 patients with and without suspected heparin-induced thrombocytopenia (HIT), vaccine-induced immune thrombotic thrombocytopenia (VITT) patients, and in controls and SID patients following COVID-19 vaccination.
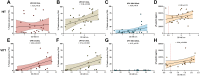
Results: No significant increase in anti-PF4/H antibody levels was observed during COVID-19 regardless of disease severity. Despite a 2-fold increase in HIT suspicion observed during the pandemic, there was no corresponding increase in HIT diagnoses. Additionally, no significant increase in anti-PF4/H levels was noted after vaccination, even in SID patients. None of the positive anti-PF4/H antibodies detected in COVID-19 or vaccination cohorts induced platelet activation, measured by soluble P-selectin levels and flow cytometry-based on platelet microvesicle generation. Finally, in VITT patients, unlike in HIT patients, anti-PF4/H levels were strongly associated with platelet microvesicle assay and moderately with soluble P-selectin levels.
Conclusion: Our study found no significant increase in anti-PF4/H antibodies in COVID-19 or after vaccination, including in SID patients. However, in VITT patients, but not in HIT patients, these antibodies were correlated with platelet activation. This finding suggests that anti-PF4/H antibodies play a different role in the pathophysiology of VITT but that their interest is limited outside clear contexts of HIT/VITT suspicion.
Keywords: COVID-19; antibodies; connectivite tissue disease; thrombocytopenia; vaccine; vasculitis.
© 2025 The Author(s).
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

