Iron content of glioblastoma tumours and role of ferrous iron in the hypoxic response in vitro
- PMID: 40123902
- PMCID: PMC11925887
- DOI: 10.3389/fonc.2025.1536549
Iron content of glioblastoma tumours and role of ferrous iron in the hypoxic response in vitro
Abstract
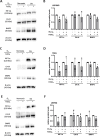
Introduction: Glioblastomas are an aggressive primary brain cancer, characterised by hypoxia and poor patient survival. Iron is the most abundant transition metal in the brain, yet data on the iron content of brain cancers is sparse. Ferrous iron is an essential cofactor for a super-family of enzymes, the iron- and 2-oxoglutarate-dependent dioxygenase enzymes (2-OGDD). These enzymes control the response to hypoxia via hydroxylation of the hypoxia-inducible factor-1α (HIF-1α), and DNA demethylation via hydroxylation of 5-methyl cytosines (5hmC).
Methods: This study used clinical glioblastoma samples from 40 patients to determine the relationship between 2-OGDD activity and iron. Elemental iron was measured using inductively coupled plasma mass spectrometry (ICP-MS) and ferrous iron was measured using the colorimetric ferrozine assay. Iron measurements were compared against patient survival and clinicopathological data, and 2-OGDD-dependent activity of HIF-1 activation and 5hmC.
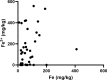
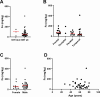
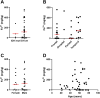
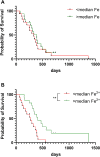
Results and discussion: Elemental and ferrous iron levels were weakly related. Higher ferrous iron content of clinical glioblastoma tissue was associated with longer overall survival compared to lower ferrous iron content, but elemental iron showed no such relationship. Neither form of iron was related to clinicopathological data or markers of 2-OGDD activity. The impact of iron supplementation on the hypoxic response was assessed in three glioblastoma cell lines in vitro, similarly showing only a limited influence of iron on these 2-OGDD enzymes. Our data, together with prior studies in anaemic patients, highlight the importance of healthy iron levels in patients with glioblastoma, but further mechanistic studies are needed to elucidate the molecular pathways involved.
Keywords: brain cancer; elemental iron; ferrous iron; glioblastoma; hypoxia; survival.
Copyright © 2025 Praditi, Beverley-Stone, Reid, Burgess, Crake, Vissers, Royds, Slatter, Dachs and Phillips.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
LinkOut - more resources
Full Text Sources

