Effect of different cell culture media on the production and glycosylation of a monoclonal antibody from a CHO cell line
- PMID: 40124126
- PMCID: PMC11928345
- DOI: 10.1007/s10616-025-00733-7
Effect of different cell culture media on the production and glycosylation of a monoclonal antibody from a CHO cell line
Abstract
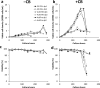
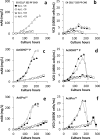
Recombinant monoclonal antibodies (mAbs) are commonly produced using Chinese hamster ovary (CHO) cells and the cell culture medium used in bioreactors influences the yield and quality attributes of the protein drug products. The COVID 19 pandemic revealed a vulnerability in the supply chain for necessary reagents (such as culture medium and raw material) for maintaining un-interrupted production of protein drugs with consistent quality. The supply interruption for the cell culture medium ActiPro™ optimized for producing VRC01, an IgG1-κ mAb, from a CHO-K1 cell line, necessitated the search for alternate media. VRC01 mAb is highly glycosylated and can broadly neutralize several strains of Human Immunodeficiency Virus (HIV). We investigated to see if an alternate medium can be used in the production without impacting quality attributes like glycosylation. In our strategy, we used 3 different commercially available media, performed two sets of experiments-with and without media supplements, Cell boost 7a and Cell boost 7b. Cell growth, volumetric production of the mAb protein and glycosylation pattern were compared to identify an alternative medium. Among the tested media based on cell growth, mAb production potential and glycosylation analysis, ActiCHO™ P was found to be a better alternate medium to ActiPro™ medium than EX-CELL® 325 PF CHO medium to produce VRC01 mAb. Overall, the approach used here to establish the impact of variation in medium on protein therapeutic attributes may be used during product development to build in supply chain resilience in drug manufacturing.
Supplementary information: The online version contains supplementary material available at 10.1007/s10616-025-00733-7.
Keywords: Chemically defined medium; Glycosylation; Monoclonal antibody (mAb); N-glycans; Titer.
© This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply 2025.
Conflict of interest statement
Conflict of interestAll authors declare that there are no conflicts of interest.
Figures


References
-
- Barrett S, Boniface R, Dhulipala P, Slade P, Tennico Y, Stramaglia M, Lio P, Gorfien S (2012) Attaining next level titers in CHO fed-batch cultures. Bioproc Int 10:56
-
- Bork K, Horstkorte R, Weidemann W (2009) Increasing the sialylation of therapeutic glycoproteins: the potential of the sialic acid biosynthetic pathway. J Pharm Sci 98(10):3499–3508. 10.1002/jps.21684 - PubMed
-
- Broedel SE, Papciak SM (2003) The case for serum-free media. BioProcess International(February) p 56–58
LinkOut - more resources
Full Text Sources

