Soluble TREM2 reflects liver fibrosis status and predicts postoperative liver dysfunction after liver surgery
- PMID: 40124168
- PMCID: PMC11929072
- DOI: 10.1016/j.jhepr.2024.101226
Soluble TREM2 reflects liver fibrosis status and predicts postoperative liver dysfunction after liver surgery
Abstract
Background & aims: Triggering receptor expressed on myeloid cells 2 (TREM2)-expressing macrophages and systemic levels of soluble TREM2 (sTREM2) appear critical in the development of chronic liver disease (CLD) and seem relevant in its detection. The aim of this study was to examine sTREM2 as a marker for early CLD and its potential to predict posthepatectomy liver failure (PHLF) in patients undergoing partial hepatectomy.
Methods: sTREM2 was assessed in the plasma of 108 patients undergoing liver resection. Blood was drawn prior to surgery (preop) and on the first and fifth postoperative day.
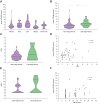
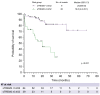
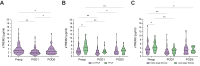
Results: Preop sTREM2 levels were similar across different indications for resection (p = 0.091). Higher preop sTREM2 levels were associated with advanced hepatic fibrosis (p = 0.030) and PHLF (p = 0.007). Fibrosis-4 index (FIB-4) (p = 0.619) and model for end-stage liver disease (MELD) (p = 0.590) did not show a difference between patients grouped by their CLD. Comparing the AUC from receiver-operating characteristic analysis, sTREM2 (AUC = 0.708) outperformed FIB-4 (AUC = 0.529), MELD (AUC = 0.587), Child-Pugh grading (AUC = 0.570) and LiMAx (liver maximum capacity test) (AUC = 0.516) in predicting PHLF. Similarly, in uni- and multivariate analysis, only sTREM2 proved predictive for PHLF (p = 0.023). High-risk (p = 0.003) and low-risk (p = 0.011) cut-offs for systemic sTREM2 levels could identify patients at risk for adverse outcomes after surgery. Finally, high sTREM2 was associated with decreased overall survival after liver surgery (p <0.001).
Conclusions: Circulating sTREM2 shows sensitivity for early-stage, asymptomatic liver disease, irrespective of the underlying indication for liver surgery. Assessment of CLD via sTREM2 monitoring could improve early detection of CLD and improve outcomes after liver surgery.
Impact and implications: Soluble TREM2 (sTREM2) has previously been shown to correlate with the degree of chronic liver disease. We found that even in patients undergoing liver resection, who generally do not suffer from end-stage liver disease, sTREM2 reflects liver fibrosis status and predicts postoperative development of liver dysfunction. This is especially relevant for liver surgeons and patients, as postoperative liver dysfunction is the main reason for postoperative mortality. Our findings are also important for hepatologists, as early detection of liver fibrosis and cirrhosis is paramount for overall patient survival and we can show that even in a cohort with a median model for end-stage liver disease score of 6, sTREM2 is able to distinguish patients based on their liver fibrosis status.
Keywords: Cirrhosis; Liver fibrosis; Liver regeneration; Liver surgery; Soluble TREM2.
© 2024 The Author(s).
Conflict of interest statement
None of the authors have any type of conflict of interest to declare in regards to the writing of this manuscript. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




References
-
- Younossi Z., Tacke F., Arrese M., et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682. - PubMed
-
- Israelsen M., Torp N., Johansen S., et al. MetALD: new opportunities to understand the role of alcohol in steatotic liver disease. Lancet Gastroenterol Hepatol. 2023;8(10):866–868. - PubMed
-
- Harrison S.A., Bedossa P., Guy C.D., et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390(6):497–509. - PubMed
LinkOut - more resources
Full Text Sources

