The future of islet transplantation beyond the BLA approval: challenges and opportunities
- PMID: 40124184
- PMCID: PMC11925927
- DOI: 10.3389/frtra.2025.1522409
The future of islet transplantation beyond the BLA approval: challenges and opportunities
Abstract
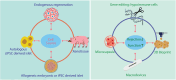
This opinion paper explores the path forward for islet transplantation as a cell therapy for type 1 diabetes, following the Biologics License Application (BLA) approval. The authors review key challenges and opportunities that lie ahead. After a brief overview of the history of human islet transplantation, the paper examines the FDA's regulatory stance on isolated islet cells and the requirements for obtaining a BLA. The authors discuss the significance of this approval and the critical steps necessary to broaden patient access, such as scaling up production, clinical integration, reimbursement frameworks, post-marketing surveillance, and patient education initiatives. The paper highlights that the approval of LANTIDRA as an allogeneic cell transplant for uncontrolled type 1 diabetes marks the beginning of new chapters in improving islet transplantation. The authors emphasize essential areas for development, including advancements in islet manufacturing, optimization of transplant sites, islet encapsulation, exploration of unlimited cell sources, and gene editing technologies. In conclusion, the future of islet transplantation beyond the BLA approval presents challenges and opportunities. While significant regulatory milestones have been reached, hurdles remain. Innovations in stem cell-derived islets, cell encapsulation, and gene editing show promise in enhancing graft survival, expanding the availability of transplantable cells, and reducing the reliance on immunosuppressive drugs. These advancements could pave the way for more accessible, durable, and personalized diabetes treatments.
Keywords: LANTIDRA; biologics license application (BLA); federal food and drug administration; islet transplant; type I diabetes.
© 2025 Wang, McGarrigle, Cook, Rios, Monica, Chen, Wei and Oberholzer.
Conflict of interest statement
Authors YW, JM, JC, PR, GM and JO were employed by company CellTrans, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources