Dataset of total emissivity for CO2, H2O, and H2O-CO2 mixtures; over a temperature range of 300-2900 K and a pressure-pathlength range of 0.01-50 atm.m
- PMID: 40124289
- PMCID: PMC11930170
- DOI: 10.1016/j.dib.2025.111428
Dataset of total emissivity for CO2, H2O, and H2O-CO2 mixtures; over a temperature range of 300-2900 K and a pressure-pathlength range of 0.01-50 atm.m
Abstract
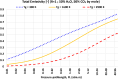
This article describes a dataset of total (spectrally-integrated rather than wavelength-dependent) emissivity values pertaining to gaseous media containing carbon dioxide (CO2) and/or water vapor (H2O) with the sum of their partial pressures being near the atmospheric level. These conditions may be particularly relevant to flue gases resulting from oxy-fuel combustion. The emissivities here are computed using the EM2C implementations of the statistical narrow band (SNB), and they are made conveniently available as 10 separate plain text files having a unified layout. Each data file in the dataset corresponds to a specific chemical composition (from pure CO2 to pure H2O), with eight intermediate H2O:CO2 molar ratios being 1:20, 1:8, 1:4, 1:2, 1:1, 2:1, 5:1, and 20:1. In addition, pure CO2 corresponds to the extreme lower-bound for the H2O:CO2 molar (as 0:1), and pure H2O corresponds to the extreme upper-bound for the H2O:CO2 molar ratio (1:0 or infinity). For each chemical composition, the total emissivity values are provided for different pressure-pathlengths and different absolute gas temperatures as two independent variables. The pressure-pathlength range spans about three orders of magnitude, from 0.01 atm.m to 50 atm.m, with 90 nonuniformly-distributed pressure-pathlength values. The absolute gas temperature spans a wide range from 300 K (room temperature) to 2900 K (high-temperature flames) with a uniform step of 25 K separating 105 temperature values. For each data file, there are 90 × 105 (9450) emissivity values; and the entire dataset contains 94500 emissivity values.
Keywords: CO2; Emissivity; H2O; Oxy-fuel; Pressure-pathlength.
© 2025 The Author(s).
Figures



References
-
- [Laboratoire Énergetique Moléculaire et Macroscopique EM2C Combustion], EM2C │ Publications, (2025). https://em2c.centralesupelec.fr/en/Publications (accessed January 14, 2025).
-
- Modest M.F. Academic Press; Amsterdam Boston: 2003. Radiative Heat Transfer, 2nd ed.
-
- Marzouk O.A. Radiant heat transfer in nitrogen-free combustion environments. Int. J. Nonlinear Sci. Numer. Simul. 2018;19:175–188. doi: 10.1515/ijnsns-2017-0106. - DOI
-
- O. Marzouk, Mendeley Data dataset │ Emissivity_CO2-H2O_94500-values_EM2C, (2025). 10.17632/X5WJZK6SJS.1. - DOI
-
- Marzouk O.A. Technical review of radiative-property modeling approaches for gray and nongray radiation, and a recommended optimized WSGGM for CO2/H2O-enriched gases. Results. Eng. 2025;25 doi: 10.1016/j.rineng.2025.103923. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

