Stiff extracellular matrix activates the transcription factor ATF5 to promote the proliferation of cancer cells
- PMID: 40124511
- PMCID: PMC11928855
- DOI: 10.1016/j.isci.2025.112057
Stiff extracellular matrix activates the transcription factor ATF5 to promote the proliferation of cancer cells
Abstract
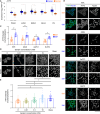
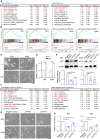
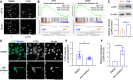
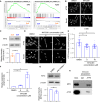
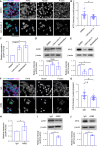
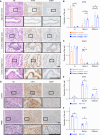
Cancer tissues are stiffer than normal tissues. Carcinogenesis stiffens the extracellular matrix (ECM) of cancerous tissues, to which cancer cells respond by activating transcription factors, such as YAP/TAZ, Twist1, and β-catenin, which further elevate their malignancy. However, these transcription factors are also expressed in normal tissues. Therefore, inhibiting these factors in order to treat cancer may lead to severe side effects. Here, we show that activating transcription factor 5 (ATF5), highly expressed in tumors, is activated by ECM stiffness and promotes the proliferation of cancer cells, including that of pancreatic cancer cells and lung cancer cells. In addition, ATF5 suppressed the expression of early growth response 1 (EGR1), thereby accelerating cancer cell proliferation. Stiff ECMs trigger the JAK-MYC pathway which activates ATF5. JAK activation was actomyosin independent whereas MYC induction was actomyosin dependent. These results demonstrate the critical role played by ATF5 in the mechanotransduction process seen in cancers.
Keywords: Biophysics; Cancer; Microenvironment.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- García-Palmero I., Torres S., Bartolomé R.A., Peláez-García A., Larriba M.J., Lopez-Lucendo M., Peña C., Escudero-Paniagua B., Muñoz A., Casal J.I. Twist1-induced activation of human fibroblasts promotes matrix stiffness by upregulating palladin and collagen α1(VI) Oncogene. 2016;35:5224–5236. doi: 10.1038/onc.2016.57. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

