Aging triggers mitochondrial, endoplasmic reticulum, and metabolic stress responses in the heart
- PMID: 40124955
- PMCID: PMC11928159
- DOI: 10.20517/jca.2024.17
Aging triggers mitochondrial, endoplasmic reticulum, and metabolic stress responses in the heart
Abstract
Introduction: Aging is a multifaceted biological process characterized by a progressive decline in cellular and tissue function. It significantly impacts the cardiovascular system and contributes to the onset of cardiovascular diseases. The mitochondria (mt) and the endoplasmic reticulum (ER) play synergistic roles in maintaining cellular homeostasis and energy production in the heart. Nevertheless, their response to cardiac aging is not well known.
Aim: This study explores mt and ER stress responses and their associated factors, such as metabolic, cellular, and autophagic stress, in cardiac aging.
Methods and results: We utilized 10- and 25-month-old CBA/CaJ mice to evaluate mt, ER, and their associated factors, such as metabolic, cellular, and autophagic stress responses. We studied the gene expression for mitochondrial biogenesis, mt and ER stress response, autophagy and metabolic markers, and activating transcription factors that mediate cellular stress responses. We found no significant difference in mtDNA content and the mRNA expression of the mt transcription factor, Tfam; however, selective mtDNA genes, such as mt-Cytb and mt-Co2, showed significant induction in 25-month-aged compared to 10-month-young hearts. Interestingly, genes of several mitochondrial stress response proteases and their components, including Lonp1, Yme1l1, Afg3l2, and Spg7, were significantly induced, with a substantial induction of Clpp and Clpx. However, age-associated differences were not observed in the induction of mt chaperones (Hspa9 and Hspd1), but significant induction of Dnaja2, a mitochondrial co-chaperone, was observed. The ER stress transcription factors Xbp1 and Atf6 were markedly induced in aged hearts, accompanied by decreased expression of ER stress chaperone Hsp90b with no change in Hspa5 and Dnajb9 chaperones. However, induction of Dnm1l was significant, whereas Mfn1 and Fis1 were downregulated in contrast to Mfn2, suggesting dysregulated mitochondrial dynamics in the aged heart with no change in autophagy and metabolic stress regulators observed. Furthermore, aged hearts showed significantly increased oxidative damage as evidenced by elevated lipid peroxidation (4-HNE) levels.
Conclusion: These findings demonstrate that aging triggers mt, ER, and oxidative stress in the heart, which over time leads to the accumulation of oxidative damage, causing cellular impairment, highlighting these pathways as potential therapeutic targets for mitigating age-related cardiac dysfunction.
Keywords: Aging; endoplasmic reticulum stress; heart; mitochondrial stress; oxidative stress.
Conflict of interest statement
Conflicts of interest All authors declared that there are no conflicts of interest.
Figures




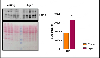
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
