In vitro and in vivo efficacy of the Active Oligo Skin complex™, a new active ingredient processed from seawater, on multiple parameters of atopic skin
- PMID: 40125013
- PMCID: PMC11924379
- DOI: 10.1093/skinhd/vzae029
In vitro and in vivo efficacy of the Active Oligo Skin complex™, a new active ingredient processed from seawater, on multiple parameters of atopic skin
Abstract
Background: Different symptoms are associated with atopic skin, including dryness, pruritus and pain, and affect patients' quality of life. The environment, microbiota, epidermis, immune and nerve cells are all implicated in the pathogenesis of atopic skin. Staphylococcus aureus is the focus of particular attention. Epidermis is implicated at multiple levels: inflammatory process, barrier, control of moisture and water loss. Sensory neurons that participate in cutaneous neurogenic inflammation and pruritus are seen as a potential new target. Specific management strategies and new treatments for adults and children are needed to help in more refractory cases. As a baseline of management, guidelines recommend a treatment to moisturize the skin and maintain the skin barrier function, such as an emollient.
Objectives: To evaluate a new product in vitro and in vivo in order to validate the potential of its use in people with atopic skin or dry skin.
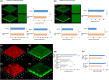
Methods: A specific mineral composition, Active Oligo Skin complex™, from seawater was developed and included in a balm. The effects of a solution and balm containing the complex were evaluated in vitro on the growth and biofilm formation of Staphylococcus aureus and Staphylococcus epidermidis in different skin models, and in vivo in adult and young volunteers.
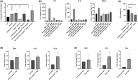
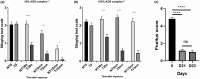
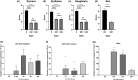
Results: In vitro, the complex modulated bacterial biofilm formation and growth, decreased cytokine [interleukin (IL)-1, IL-6, IL-4] and neuropeptide (substance P) release, and increased the expression of CL1 and CL4. On volunteers with dry skin, the complex had a moisturizing effect after 1 h of application. Dryness and roughness were also reduced in young participants with atopic skin. The balm decreased erythema and pruritus after 21 days of topical application on 60 young participants. On 22 adult participants, stinging score was decreased after -application.
Conclusions: The Active Oligo Skin complex™ appears to display potent antipruritic and anti-inflammatory activities, both in vitro and in vivo.
© The Author(s) 2025. Published by Oxford University Press on behalf of British Association of Dermatologists.
Conflict of interest statement
Conflict of interest: N.L., C.C., M.G.F. and L.M. have a conflict of interest with Laboratoires Gilbert. E.S., S.C. and J.G. are employees of Laboratoires Gilbert.
Figures
Similar articles
-
Effects of a New Emollient-Based Treatment on Skin Microflora Balance and Barrier Function in Children with Mild Atopic Dermatitis.Pediatr Dermatol. 2016 Mar-Apr;33(2):165-71. doi: 10.1111/pde.12786. Pediatr Dermatol. 2016. PMID: 27001317 Free PMC article. Clinical Trial.
-
Blockade of interleukin-13 signalling improves skin barrier function and biology in patients with moderate-to-severe atopic dermatitis.Br J Dermatol. 2024 Aug 14;191(3):344-350. doi: 10.1093/bjd/ljae138. Br J Dermatol. 2024. PMID: 38531691
-
Hygiene and emollient interventions for maintaining skin integrity in older people in hospital and residential care settings.Cochrane Database Syst Rev. 2020 Jan 23;1(1):CD011377. doi: 10.1002/14651858.CD011377.pub2. Cochrane Database Syst Rev. 2020. PMID: 32006460 Free PMC article.
-
A Role of Staphyococcus aureus, Interleukin-18, Nerve Growth Factor and Semaphorin 3A, an Axon Guidance Molecule, in Pathogenesis and Treatment of Atopic Dermatitis.Allergy Asthma Immunol Res. 2010 Oct;2(4):235-46. doi: 10.4168/aair.2010.2.4.235. Epub 2010 May 12. Allergy Asthma Immunol Res. 2010. PMID: 20885908 Free PMC article.
-
Interventions to reduce Staphylococcus aureus in the management of eczema.Cochrane Database Syst Rev. 2019 Oct 29;2019(10):CD003871. doi: 10.1002/14651858.CD003871.pub3. Cochrane Database Syst Rev. 2019. PMID: 31684694 Free PMC article.
References
-
- De Benedetto A, Boguniewicz M, Ong PY et al. Atopic dermatitis (eczema) guidelines 2023: highlights. J Allergy Clin Immunol Pract 2024; 12:2955–65. - PubMed
-
- Demessant-Flavigny AL, Connétable S, Kerob D et al. Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: a narrative review. J Eur Acad Dermatol Venereol 2023; 37(Suppl 5):3–17. - PubMed
-
- Gouin O, Lebonvallet N, L’Herondelle K et al. Self-maintenance of neurogenic inflammation contributes to a vicious cycle in skin. Exp Dermatol 2015; 24:723–6. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
