External stimuli-responsive drug delivery to the posterior segment of the eye
- PMID: 40126105
- PMCID: PMC11934192
- DOI: 10.1080/10717544.2025.2476140
External stimuli-responsive drug delivery to the posterior segment of the eye
Abstract
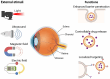
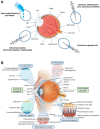
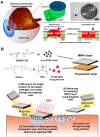
Posterior segment eye diseases represent the leading causes of vision impairment and blindness globally. Current therapies still have notable drawbacks, including the need for frequent invasive injections and the associated risks of severe ocular complications. Recently, the utility of external stimuli, such as light, ultrasound, magnetic field, and electric field, has been noted as a promising strategy to enhance drug delivery to the posterior segment of the eye. In this review, we briefly summarize the main physiological barriers against ocular drug delivery, focusing primarily on the recent advancements that utilize external stimuli to improve treatment outcomes for posterior segment eye diseases. The advantages of these external stimuli-responsive drug delivery strategies are discussed, with illustrative examples highlighting improved tissue penetration, enhanced control over drug release, and targeted drug delivery to ocular lesions through minimally invasive routes. Finally, we discuss the challenges and future perspectives in the translational research of external stimuli-responsive drug delivery platforms, aiming to bridge existing gaps toward clinical use.
Keywords: Drug delivery; external stimuli; nanomedicines; photoresponsive nanoparticles; posterior segment eye diseases.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Formulation Advances in Posterior Segment Ocular Drug Delivery.J Ocul Pharmacol Ther. 2025 Apr;41(3):101-130. doi: 10.1089/jop.2024.0153. Epub 2025 Jan 23. J Ocul Pharmacol Ther. 2025. PMID: 39842469 Review.
-
Depot formulations to sustain periocular drug delivery to the posterior eye segment.Drug Discov Today. 2019 Aug;24(8):1458-1469. doi: 10.1016/j.drudis.2019.03.023. Epub 2019 Mar 28. Drug Discov Today. 2019. PMID: 30930148 Review.
-
Implants for drug delivery to the posterior segment of the eye: a focus on stimuli-responsive and tunable release systems.J Control Release. 2014 Dec 28;196:208-21. doi: 10.1016/j.jconrel.2014.09.030. Epub 2014 Oct 13. J Control Release. 2014. PMID: 25307997 Review.
-
A review of nanocarrier-mediated drug delivery systems for posterior segment eye disease: challenges analysis and recent advances.J Drug Target. 2021 Aug;29(7):687-702. doi: 10.1080/1061186X.2021.1878366. Epub 2021 Feb 1. J Drug Target. 2021. PMID: 33474998 Review.
-
Light-responsive in situ forming injectable implants for effective drug delivery to the posterior segment of the eye.Expert Opin Drug Deliv. 2016 Jul;13(7):953-62. doi: 10.1517/17425247.2016.1163334. Epub 2016 Mar 24. Expert Opin Drug Deliv. 2016. PMID: 26967153 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
