ISG15 Enhances the Activity of γ-Glutamate Cysteine Ligase to Suppress Apoptosis in High Fat Diet-Promoted Hepatocellular Carcinoma
- PMID: 40126377
- PMCID: PMC12097128
- DOI: 10.1002/advs.202416401
ISG15 Enhances the Activity of γ-Glutamate Cysteine Ligase to Suppress Apoptosis in High Fat Diet-Promoted Hepatocellular Carcinoma
Abstract
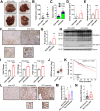
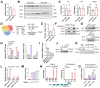
Obesity is a leading risk factor for development of hepatocellular carcinoma (HCC). High-fat intake produces cytotoxic effects in liver cells, such as excessive reactive oxygen species (ROS) accumulation and apoptosis. How HCC cells regulate ROS level and escape the cytotoxic effects of high fat diet (HFD) stress remains unclear. Herein, this work reports a critical anti-ROS/apoptotic role of the ubiquitin-like protein interferon stimulated gene 15 (ISG15) in HFD-promoted HCC. In mouse models and clinical HCC samples, upregulation of ISG15 is associated with hepatic steatosis. Notably, upregulated ISG15 elevates cellular glutathione levels, which subsequently reduces ROS accumulation and confers resistance to apoptosis in HCC cells. In diethylnitrosamine-induced HCC mouse model, HFD-feeding promotes HCC progression in wildtype mice, while tumor growth is significantly suppressed accompanied by apoptosis of HCC cells in Isg15-KO mice. Mechanistically, ISG15 promotes the activity of γ-glutamate cysteine ligase (γ-GCL), a rate-limiting heterodimeric holoenzyme of glutathione synthesis consisting of glutamate-cysteine ligase catalytic subunit (GCLC) and glutamate-cysteine ligase modifier subunit (GCLM). Independent of ISGylation, ISG15 forms an ISG15/GCLM/GCLC complex that promotes GCLM-GCLC interaction, increases glutathione generation and inhibits HFD-induced apoptosis in HCC cells. Together, an anti-apoptotic ISG15-γ-GCL-glutothione axis is suggested in HFD-promoted HCC.
Keywords: glutathione; hepatocellular carcinoma; high fat diet; interferon stimulated gene 15; reactive oxygen species; γ‐glutamate cysteine ligase.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Interferon-stimulated gene 15 (ISG15) is a trigger for tumorigenesis and metastasis of hepatocellular carcinoma.Oncotarget. 2014 Sep 30;5(18):8429-41. doi: 10.18632/oncotarget.2316. Oncotarget. 2014. PMID: 25238261 Free PMC article.
-
Differential regulation of glutamate-cysteine ligase subunit expression and increased holoenzyme formation in response to cysteine deprivation.Biochem J. 2006 Jan 1;393(Pt 1):181-90. doi: 10.1042/BJ20051111. Biochem J. 2006. PMID: 16137247 Free PMC article.
-
Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels.J Biol Chem. 2005 Oct 7;280(40):33766-74. doi: 10.1074/jbc.M504604200. Epub 2005 Aug 4. J Biol Chem. 2005. PMID: 16081425
-
Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase.Mol Aspects Med. 2009 Feb-Apr;30(1-2):86-98. doi: 10.1016/j.mam.2008.08.009. Epub 2008 Sep 6. Mol Aspects Med. 2009. PMID: 18812186 Free PMC article. Review.
-
Modulating GSH synthesis using glutamate cysteine ligase transgenic and gene-targeted mice.Drug Metab Rev. 2008;40(3):465-77. doi: 10.1080/03602530802186587. Drug Metab Rev. 2008. PMID: 18642143 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
