Reldesemtiv in Amyotrophic Lateral Sclerosis: Results From the COURAGE-ALS Randomized Clinical Trial
- PMID: 40126464
- PMCID: PMC11933997
- DOI: 10.1001/jamaneurol.2025.0241
Reldesemtiv in Amyotrophic Lateral Sclerosis: Results From the COURAGE-ALS Randomized Clinical Trial
Abstract
Importance: Treatment options for amyotrophic lateral sclerosis (ALS) remain suboptimal. Results from a phase 2 study of reldesemtiv in ALS suggested that it may slow disease progression.
Objective: To assess the effect of reldesemtiv vs placebo on functional outcomes in ALS.
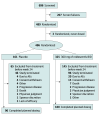
Design, setting, and participants: A Study to Evaluate the Efficacy and Safety of Reldesemtiv in Patients With Amyotrophic Lateral Sclerosis (COURAGE-ALS) was a double-blind, placebo-controlled phase 3 randomized clinical trial conducted at 83 ALS centers in 16 countries from August 2021 to July 2023. The first 24-week period was placebo controlled vs reldesemtiv. All participants received reldesemtiv during the second 24-week period with a 4-week follow-up. Two interim analyses were planned, the first for futility and the second for futility and possible resizing. This was a hybrid decentralized trial with approximately half the trial visits performed remotely and the remaining visits in the clinic. Eligible participants met criteria for definite, probable, or possible ALS with lower motor neuron signs by modified El Escorial Criteria, ALS symptoms for 24 months or less, ALS Functional Rating Scale-Revised (ALSFRS-R) total score of 44 or less, and forced vital capacity of greater than or equal to 65% of predicted.
Interventions: Oral reldesemtiv, 300 mg, or placebo twice daily.
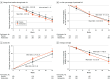
Main outcomes and measures: The primary end point was change in ALSFRS-R total score from baseline to week 24.
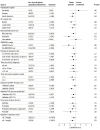
Results: Of the 696 participants screened, 207 were screen failures. A total of 486 participants (mean [SD] age, 59.4 [10.9] years; 309 male [63.6%]) were randomized to reldesemtiv (n = 325) or placebo (n = 161); 3 randomized patients were not dosed. The second interim analysis at 24 weeks after randomization included 256 participants. The data monitoring committee recommended that the trial should end due to futility, and the sponsor agreed. The mean (SE) group difference in the ALSFRS-R score from baseline to week 24 was -1.1 (0.53; 95% CI, -2.17 to -0.08; P = .04, favoring placebo). Given excess missing data from early termination, the combined assessment assumed greater importance; it, too, failed to show a benefit from treatment with reldesemtiv (win probability was 0.44 for reldesemtiv and 0.49 for placebo, with a win ratio of 0.91; 95% CI of win ratio, 0.77-1.10; P = .11).
Conclusions and relevance: This randomized clinical trial failed to demonstrate efficacy for reldesemtiv in slowing functional decline in ALS.
Trial registration: ClinicalTrials.gov Identifier: NCT04944784.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

