Hematological toxicity of [225Ac]Ac-PSMA-617 and [177Lu]Lu-PSMA-617 in RM1-PGLS syngeneic mouse model
- PMID: 40126733
- PMCID: PMC11933494
- DOI: 10.1186/s41181-025-00333-y
Hematological toxicity of [225Ac]Ac-PSMA-617 and [177Lu]Lu-PSMA-617 in RM1-PGLS syngeneic mouse model
Abstract
Background: Prostate cancer (PC) has a 34% 5-year survival rate after progressing to metastatic castration-resistant prostate cancer (mCRPC), which occurs in 20-30% of cases. Treatments like chemotherapy, immunotherapy, and PSMA-targeted radioligand therapy (RLT) show promise, but challenges remain with tumor resistance, side effects, and dose-limiting toxicity in kidneys and bone marrow. This study investigated the hematotoxicity, treatment efficacy, and recovery after [177Lu]Lu-PSMA-617 and [225Ac]Ac-PSMA-617 treatment in a syngeneic PC mouse model.
Method: Twenty-five male C57BL/6 mice were inoculated with RM1-PGLS cells and monitored using [68Ga]Ga-PSMA-11 PET/CT. The mice were divided into five groups as follows: (1) [225Ac]Ac-PSMA-617 treatment with tumors, (2) [177Lu]Lu-PSMA-617 treatment with tumors, (3) control group with tumors, (4) [225Ac]Ac-PSMA-617 treatment without tumors, and (5) [177Lu]Lu-PSMA-617 treatment without tumors. Tumor volume was measured weekly, and animals were sacrificed when tumors reached 1.5 cm³. Endpoint criteria included tumor size, survival, and body mass. Blood samples were collected at different time points to assess blood cell counts and liver and kidney function.
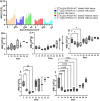
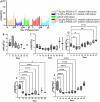
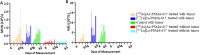
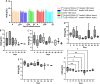
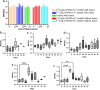
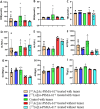
Results: Both treatments significantly slowed tumor progression and extended survival. [225Ac]Ac-PSMA-617-treated mice had a median survival of 70 days, compared to 58 days for [177Lu]Lu-PSMA-617-treated mice and 30 days for the control group. Tumor volumes were significantly reduced in both treatment groups (P < 0.05). Hematological analysis showed that both treatments reduced WBCs, RBCs, and platelets, but values normalized within 35-42 days. Liver and kidney functions remained unaffected, and no significant renal or hepatic toxicity was observed.
Conclusion: Both [225Ac]Ac-PSMA-617 and [177Lu]Lu-PSMA-617 caused transient hematotoxicity without prolonged effects. The data do not explicitly support the necessity of immunocompetent models for studying therapeutic outcomes in this context. Future studies incorporating immune profiling are warranted to investigate immune system interactions in radioligand therapy further.
Keywords: Actinium-225; Hematotoxicity; Luetitium-177; PC; PSMA-617; RLT.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: All animal studies were approved by the UCLA Institutional Animal Care and Use Committee, known as the Chancellor’s Animal Research Committee (ARC; # 2005-090). The institutional guidelines for the care and use of animals were strictly followed. Twenty-five mice were housed under pathogen-free conditions. Water and food were provided ad libitum. Male C57BL/6 mice were injected subcutaneously with into the shoulder region. Tumor growth was monitored by caliper. Animals were sacrificed upon reaching any of the termination criteria specified in the ARC protocol, including but not limited to apathy, ulceration, severe weight loss, or other signs of deteriorating condition. The study was carried out in compliance with the ARRIVE guidelines. This article does not contain any studies with human participants. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests related to this work.
Figures




References
-
- Babich JW, et al. Therapeutic efficacy of PSMA-Targeted radioligands in prostate Cancer xenograft models. Eur J Nucl Med Mol Imaging. 2020;47(5):1175–84. 10.1007/s00259-019-04556-7.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
