Respiratory Syncytial Virus Humoral Antibody Responses in Older Adults After Vaccination or Infection
- PMID: 40126899
- PMCID: PMC12247800
- DOI: 10.1093/infdis/jiaf149
Respiratory Syncytial Virus Humoral Antibody Responses in Older Adults After Vaccination or Infection
Abstract
Background: Immunity to respiratory syncytial virus (RSV) is short lived following infection. We determined if recently licensed RSV preF vaccines induce better immune responses than infection.
Methods: Serum preF binding and neutralizing antibody to RSV A and B was measured in older adults at baseline and 30 days after RSV infection or vaccination with 2 licensed RSV preF protein vaccines.
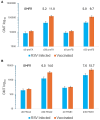
Results: Vaccination induced higher serum preF binding antibody and neutralizing responses than RSV infection (A2 RSV, 3306 vs 1254, P < .0001; B1 RSV, 5153 vs 2186, P < .0001).
Conclusions: Vaccination with preF protein vaccines induce better humoral immune responses than RSV infection in older adult.
Keywords: RSV infection; RSV preF vaccines; adults; humoral antibody response.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. E. W. has had grant support from Merck, Pfizer, and Moderna; and serves on advisory boards to Merck, Pfizer, Moderna, AstraZeneca, and GSK. A. F. has had grant support from AstraZeneca, Pfizer, and Moderna; and serves on advisory panels to Pfizer, GSK, AstraZeneca, and Moderna. A. B. has grant support from Pfizer, Moderna, and Cyanvac; and is a consultant to Moderna, GSK, and Sanofi. M. P. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. - PubMed
-
- Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med 2023; 388:595–608. - PubMed
-
- Walsh EE, Pérez Marc G, Zareba AM, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023; 388:1465–77. - PubMed
-
- Wilson E, Goswami J, Baqui AH, et al. Efficacy and safety of an mRNA-based RSV preF vaccine in older adults. N Engl J Med 2023; 389:2233–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

