Control of circadian muscle glucose metabolism through the BMAL1-HIF axis in obesity
- PMID: 40127275
- PMCID: PMC12002348
- DOI: 10.1073/pnas.2424046122
Control of circadian muscle glucose metabolism through the BMAL1-HIF axis in obesity
Abstract
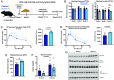
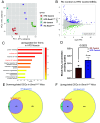
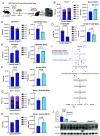
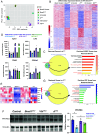
Disruptions of circadian rhythms are widespread in modern society and lead to accelerated and worsened symptoms of metabolic syndrome. In healthy mice, the circadian clock factor BMAL1 is required for skeletal muscle function and metabolism. However, the importance of muscle BMAL1 in the development of metabolic diseases, such as diet-induced obesity (DIO), remains unclear. Here, we demonstrate that skeletal muscle-specific BMAL1-deficient mice exhibit worsened glucose tolerance upon high-fat diet feeding, despite no evidence of increased weight gain. Metabolite profiling from Bmal1-deficient muscles revealed impaired glucose utilization specifically at early steps in glycolysis that dictate the switch between anabolic and catabolic glucose fate. We provide evidence that this is due to abnormal control of the nutrient stress-responsive hypoxia-inducible factor (HIF) pathway. Genetic HIF1α stabilization in muscle Bmal1-deficient mice restores glucose tolerance and expression of 217/736 dysregulated genes during DIO, including glycolytic enzymes. Together, these data indicate that during DIO, skeletal muscle BMAL1 is an important regulator of HIF-driven glycolysis and metabolic flexibility, which influences the development of high-fat-diet-induced glucose intolerance.
Keywords: circadian rhythm; diet-induced obesity; hypoxia; skeletal muscle.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Bass J., Lazar M. A., Circadian time signatures of fitness and disease. Science 354, 994–999 (2016). - PubMed
-
- Schibler U., et al. , Clock-talk: Interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb. Symp. Quant Biol. 80, 223–232 (2015). - PubMed
-
- Roenneberg T., Allebrandt K. V., Merrow M., Vetter C., Social jetlag and obesity. Curr. Biol. 22, 939–943 (2012). - PubMed
MeSH terms
Substances
Grants and funding
- F31 DK139621/DK/NIDDK NIH HHS/United States
- R01DK123358/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01 DK123358/DK/NIDDK NIH HHS/United States
- F31DK139621/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- T32GM00806140/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 AG078174/AG/NIA NIH HHS/United States
- R01HL166356/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL166356/HL/NHLBI NIH HHS/United States
- R03DK130908-01A1/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R03 DK130908/DK/NIDDK NIH HHS/United States
- R01AG078174/HHS | NIH | National Institute on Aging (NIA)
LinkOut - more resources
Full Text Sources
Medical

