Real-world outcomes for young adult patients receiving CD19 CAR T-cell therapy
- PMID: 40127395
- PMCID: PMC12166372
- DOI: 10.1182/bloodadvances.2024014846
Real-world outcomes for young adult patients receiving CD19 CAR T-cell therapy
Abstract
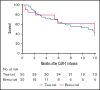
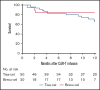
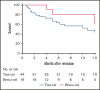
Tisagenlecleucel (tisa-cel) and brexucabtagene autoleucel (brexu-cel) are approved CD19 chimeric antigen receptor T-cell therapy (CAR T) products for young adults (YA) with relapsed/refractory B-cell acute lymphoblastic leukemia. A distinct analysis of YAs receiving commercial CD19 CAR T has not been reported. Using retrospective data from the Pediatric Real-World CAR T Consortium and the Real-World Outcomes of CAR T in Adult ALL collaboration, we describe the efficacy and safety of tisa-cel and brexu-cel in 70 YAs (18-26 years; tisa-cel, n = 50; brexu-cel, n = 20). Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were observed more frequently for brexu-cel vs tisa-cel (CRS, 85% vs 56%; ICANS, 40% vs 18%). Complete response rates were similar between products at 80% for brexu-cel and 88% for tisa-cel. Relapse-free survival (RFS) at 12 months was 46% for brexu-cel and 36% for tisa-cel. Durability of remission over 12 months was 61% for brexu-cel vs 41% for tisa-cel; 12-month overall survival (OS) for brexu-cel was 84% vs 68% for tisa-cel. In multivariate analysis, low disease burden was associated with improved OS, whereas inotuzumab before CAR T was associated with inferior outcomes. This study demonstrates comparable real-world efficacy among YAs receiving CD19 CAR T irrespective of CAR T construct; however, rates of toxicity seem higher with brexu-cel.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: L.M.S. has served on advisory committees for Novartis and Cargo Therapeutics. I.A. has served as a consultant for Kite, Sobi, Jazz, Pfizer, Amgen, and Takeda; received honoraria from Takeda; served on advisory committees for Amgen, Pfizer, Jazz, Kite, Takeda, Syndax, Sobi, and Wugen; and received research support from AbbVie and MacroGenics. J.R. has served as a consultant for Novartis. K.J.C. has served as a consultant for Novartis; served as a board member for Turn Bio and PromiCell; and received research funding from Novartis, Cellectis, and Atara Biotherapeutics. C.L.P. has served on an advisory committee for Novartis. S.H.C.B.’s spouse is employed by Takeda. S.C. has served as a consultant for Pfizer and Jazz. M.H. has served on advisory committees for Sobi and Novartis. P.S. has served on an advisory committee for Sobi. M.Q. has served as a consultant for Novartis and Mesoblast. R.D.C. has served on advisory committees for Autolus and PeproMene Bio; served as a consultant and received honoraria for Autolus, Amgen, Jazz, Kite, and Pfizer; and received research funding from Amgen, Kite, Incyte, Merck, Pfizer, Servier, and Vanda, and R.D.C.’s spouse was employed by and owned stock in Seagen. V.K.K. has received honoraria from Pfizer, Novartis, and Kite, and received research funding from Incyte. P.S. received honoraria from Autolus and Bristol Myers Squibb (BMS), and served on a speaker’s bureau for Sanofi. M.S. served as a consultant for Jazz and Novartis. A.L. served as a consultant for Pfizer and CTI BioPharma. G.Y. served on a speaker’s bureau for Jazz, Kite, BMS, Incyte, Sobi, GlaxoSmithKline, and Servier. V.B. served on advisory committees for AstraZeneca, Allogene, BeiGene, and CRISPR, and received research funding from Citius and Incyte. E.C. served as a consultant for AbbVie. A.S.A. served on advisory committees for Jazz, Taiho, and Novartis; received honoraria from Kite, Pfizer, Jazz, Beam, Nkarta, Novartis, Kura, and Amgen; and received research funding from Servier, ImmunoGen, GlycoMimetics, Pfizer, Incyte, Seattle Genetics, and MacroGenics. B.S. received research funding from Incyte, Jazz, Kite, and Servier; received honoraria from Janssen, Spectrum/Acrotech, BeiGene, and Gilead Sciences; served as a consultant for PeproMene, Lilly, Autolus, Deciphera, Century Therapeutics, Jazz, Kite, Precision Biosciences, Amgen, Pfizer, Novartis, BMS, Adaptive Biotechnologies, AstraZeneca, and Takeda; and served on an advisory committee for PeproMene Bio. L.M. served as a consultant for Amgen, Pfizer, Kite, Autolus, and Astellas; received honoraria from Kite; received research funding from BMS, Adaptive, Kite, Astellas, Orca Bio, and Jasper; and served on an advisory committee for Adaptive. R.F. received research funding from Kite and Novartis; and served on an advisory committee for Kite. The remaining authors declare no competing financial interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

