Sensing and detection performance of the novel, small-diameter OmniaSecure defibrillation lead: in-depth analysis from the LEADR trial
- PMID: 40127675
- PMCID: PMC12001238
- DOI: 10.1093/europace/euaf062
Sensing and detection performance of the novel, small-diameter OmniaSecure defibrillation lead: in-depth analysis from the LEADR trial
Abstract
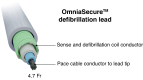
Aims: The Lead EvaluAtion for Defibrillation and Reliability (LEADR) trial evaluated the small-diameter (4.7 Fr), integrated bipolar OmniaSecure defibrillation lead. As previously reported, the trial exceeded primary safety and efficacy objective thresholds, demonstrating favourable performance and zero fractures through ∼12 months follow-up, with patients in ongoing follow-up. Longer-term follow-up of the LEADR trial with emphasis on the sensing and detection capabilities of the OmniaSecure lead is reported here.
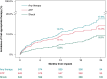
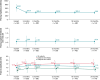
Methods and results: Patients with indications for de novo implantable cardioverter-defibrillators/cardiac resynchronisation therapy defibrillators were implanted with the OmniaSecure lead in standard right ventricle (RV) locations and followed at pre-specified intervals along with CareLink™ remote monitoring transmissions, where available. Throughout follow-up, the lead was evaluated for safety, efficacy, and reliability along with sensing and detection performance. There were 643/657 (97.9%) patients successfully implanted with the OmniaSecure lead with mean follow-up of 18.2 ± 5.5 months. There was a 96.9% freedom from major study lead-related complications at 24 months. Inappropriate shock rate was 2.7 and 3.8% at 12 and 24 months, respectively. At 24 months, 17.6% of patients received appropriate therapies (shock and/or ATP) with a 76.5% ATP efficacy. There have been zero fractures during follow-up along with chronically stable pacing capture threshold, pacing impedance, and R-wave amplitudes. There were four patients with an adverse event related to PWOS (0.6%), none of which was associated with inappropriate shock. There were four patients with an adverse event related to TWOS (0.6%), of which three patients were associated with inappropriate shock (0.5%). Oversensing was resolved predominantly by programming the RV sensitivity to less sensitive settings. During VF induction at implant, 97.6% (120/123) of patients showed appropriate VF episode detection at the least sensitive setting of 1.2 mV, with the remaining having detection at more sensitive settings. In follow-up, 670 VT/VF episodes were appropriately detected and treated in 94 patients with a variety of RV sensitivities and no reports of under-detected episodes. Moreover, a virtual sensitivity analysis also showed no under-detection across different RV sensitivity programming.
Conclusion: Chronic sensing performance of the OmniaSecure defibrillation lead demonstrated R-wave stability with a low rate of P-wave and T-wave oversensing, resolved predominantly by adjusting RV sensitivity. Further, VT/VF detection was successful and was not impacted when programmed to less sensitive settings. The OmniaSecure lead shows robust sensing and detection performance and programmability in ongoing follow-up.
Keywords: Anti-tachycardia pacing; Implantable cardioverter-defibrillator; Inappropriate shocks; Lumenless leads; P-wave oversensing.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: P.S.: reports having served on the advisory boards of Medtronic, Abbott, Boston Scientific, PaceMate, and CathRx; the University of Adelaide has received on his behalf lecture and/or consulting fees from Medtronic, Abbott, and Boston Scientific; and the University of Adelaide has received on his behalf research funding from Medtronic, Abbott, Boston Scientific, and Becton-Dickson. P.K.M.: consultant for Medtronic, Boston Scientific, and Cook as well as honoraria from Medtronic and Cook. B.H.: reports funding from Medtronic and CVRx. P.D.F.: consultant fees and travel support from Medtronic, Boston Scientific, Abbott, and Biotronik as well as honoraria and advisory board participation for Medtronic and Abbott. M.J.S.: consultant for Medtronic and Tenaya Therapeutics. D.P.S.: institutional funding from Medtronic. J.S.Z.: consultant for Medtronic. F.P.: speaker at Medtronic and Boston Scientific; consultant for Medtronic and Boston Scientific; research funding from Medtronic and Boston Scientific. B.T.: honorarium from Boston scientific. R.K.P.: reports that Canberra Heart Rhythm Foundation has received on his behalf lecture and/or consulting fees from Medtronic, Abbott Medical, Boston Scientific, and Biotronik. T.D.R.: consultant for Medtronic, Johnson and Johnson, and Philips; research funding from Medtronic and Abbott. M.F.: consultant for Medtronic. R.D.S: consultant for Medtronic as well as speaking honoraria. I.A.: consultant for Medtronic. A.M.: consultant fees and travel support from Medtronic, Boston Scientific, Abbott, Biotronik and Microport, advisory board participation for Medtronic and Boston. B.B.: consultant for Medtronic. A.E.T., K.A., B.M., and C.B.: employees of Medtronic, Inc. G.H.C.: speaker at Medtronic and Philips; consultant for Medtronic and Boston Scientific.
Figures


References
-
- Swerdlow CD, Kalahasty G, Ellenbogen KA. Implantable cardiac defibrillator lead failure and management. J Am Coll Cardiol 2016;67:1358–68. - PubMed
-
- De Filippo P, Giofre F, Leidi C, Senni M, Ferrari P. Transvenous pacing in pediatric patients with bipolar lumenless lead: ten-year clinical experience. Int J Cardiol 2018;255:45–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

