Intravenous psilocybin induces dose-dependent changes in functional network organization in rat cortex
- PMID: 40128190
- PMCID: PMC11933319
- DOI: 10.1038/s41398-025-03308-4
Intravenous psilocybin induces dose-dependent changes in functional network organization in rat cortex
Abstract
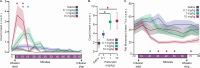
Psilocybin produces an altered state of consciousness in humans and is associated with complex spatiotemporal changes in cortical networks. Given the emphasis on rodent models for mechanistic studies, there is a need for characterization of the effect of psilocybin on cortex-wide network dynamics. Previous electroencephalographic studies of psychedelics in rodents have primarily used sparse electrode arrays with limited spatial resolution, precluding network level analysis, and have been restricted to lower gamma frequencies. Therefore, in this study, we used electroencephalographic recordings from 27 sites/electrodes across rat cortex (n = 6 male, 6 female) to characterize the effect of psilocybin (0.1, 1, and 10 mg/kg delivered over an hour) on brain network organization as inferred through changes in node degree (an index of network density) and connection strength (via weighted phase-lag index). The removal of aperiodic component from the electroencephalogram localized the primary oscillatory changes to theta (4-10 Hz), medium gamma (70-110 Hz), and high gamma (110-150 Hz) bands, which were used for the network analysis. Additionally, we determined the concurrent changes in theta-gamma phase-amplitude coupling. We report that psilocybin, in a dose-dependent manner, 1) disrupted theta-gamma coupling [p < 0.05], 2) increased frontal high gamma connectivity [p < 0.05] and posterior theta connectivity [p ≤ 0.049], and 3) increased frontal high gamma [p < 0.05] and posterior theta [p ≤ 0.046] network density. The behavioral activity and the medium gamma frontoparietal connectivity showed an inverted-U relationship with psilocybin dose. Our results suggest that high-frequency network organization, decoupled from local theta-phase, may be an important signature of psilocybin-induced non-ordinary state of consciousness.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Jim Gilligan, Ph.D. is President & Chief Scientific Officer at Tryp Therapeutics. Peter Guzzo, PhD is Consulting VP, Drug Development at Tryp Therapeutics. Ethics approval and consent to participate: All experiments were approved by the Institutional Animal Care and Use Committee at the University of Michigan, Ann Arbor, and were conducted in compliance with the Guide for the Care and Use of Laboratory Animals (Ed 8, National Academies Press) and ARRIVE Guidelines.
Figures




Update of
-
Psilocybin induces dose-dependent changes in functional network organization in rat cortex.bioRxiv [Preprint]. 2024 Feb 15:2024.02.09.579718. doi: 10.1101/2024.02.09.579718. bioRxiv. 2024. Update in: Transl Psychiatry. 2025 Mar 25;15(1):93. doi: 10.1038/s41398-025-03308-4. PMID: 38405722 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R01GM111293/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- GM111293/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01GM121919/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 GM111293/GM/NIGMS NIH HHS/United States
- R01 GM121919/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

