Portable molecular diagnostic platform for rapid point-of-care detection of mpox and other diseases
- PMID: 40128193
- PMCID: PMC11933461
- DOI: 10.1038/s41467-025-57647-3
Portable molecular diagnostic platform for rapid point-of-care detection of mpox and other diseases
Abstract
The World Health Organization's designation of mpox as a public health emergency of international concern in August 2024 underscores the urgent need for effective diagnostic solutions to combat this escalating threat. The rapid global spread of clade II mpox, coupled with the sustained human-to-human transmission of the more virulent clade I mpox in the Democratic Republic of Congo, highlights a critical gap in point-of-care diagnostics for this emergent disease. In response, we developed Dragonfly, a portable molecular diagnostic platform for point-of-care use that integrates power-free nucleic acid extraction (<5 minutes) with lyophilised colourimetric LAMP chemistry. The platform demonstrated an analytical limit-of-detection of 100 genome copies per reaction for monkeypox virus, effectively distinguishing it from other orthopoxviruses, herpes simplex virus, and varicella-zoster virus. Clinical validation on 164 samples, including 51 mpox-positive cases, yielded 96.1% sensitivity and 100% specificity for orthopoxviruses, and 94.1% sensitivity and 100% specificity for monkeypox virus. Here, we present a rapid, accessible, and robust point-of-care diagnostic solution for mpox, suitable for both low- and high-resource settings, addressing the global resurgence of orthopoxviruses in the context of declining smallpox immunity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing financial interest(s): M.L.C., K.M.C., I.P., S.M., N.M., M.C., K.M.S., K.T.M., P.G. and J.R.M. have or had financial interest in ProtonDx Ltd, which currently has exclusive license to the intellectual property linked to Dragonfly (WO2023131803A1), SmartLid (WO2022180376A1), and their associated trademarks. These authors declare that they do not have any other known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The remaining authors declare no competing interests. Ethics: Every experiment involving clinical samples has been carried out following a protocol approved by a national research ethics committee. Specifically, this study used fully anonymised surplus samples, which was approved by the West London National Research Ethics Committee (approval no. 06/Q0406/20).
Figures
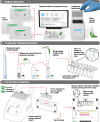
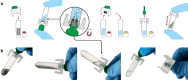



References
-
- WHO Director-General declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern. https://www.who.int/azerbaijan/news/item/23-07-2022-who-director-general....
-
- 2022-24 Mpox (Monkeypox) Outbreak: Global Trends. https://worldhealthorg.shinyapps.io/mpx_global/.
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

