A combined adjuvant and ferritin nanocage based mucosal vaccine against Streptococcus pneumoniae induces protective immune responses in a murine model
- PMID: 40128220
- PMCID: PMC11933286
- DOI: 10.1038/s41467-025-58115-8
A combined adjuvant and ferritin nanocage based mucosal vaccine against Streptococcus pneumoniae induces protective immune responses in a murine model
Erratum in
-
Author Correction: A combined adjuvant and ferritin nanocage based mucosal vaccine against Streptococcus pneumoniae induces protective immune responses in a murine model.Nat Commun. 2025 Apr 1;16(1):3129. doi: 10.1038/s41467-025-58562-3. Nat Commun. 2025. PMID: 40169610 Free PMC article. No abstract available.
Abstract
Protein nanocages are multimeric structures that can be engineered to mimic the molecular conformation of microorganisms. Based on previous findings showing that a mucosal FlaB-tPspA fusion (flagellin fused with truncated PspA antigen of Streptococcus pneumoniae) vaccine-induced protective immune response against S. pneumoniae, we develop a ferritin nanocage vaccine displaying multivalent presentation of both antigen and adjuvant on a nanocarrier using the SpyTag/SpyCatcher strategy. The 1:1 antigen/adjuvant nanocage is further used as a mucosal vaccine, which can translocate to draining lymph nodes with higher efficiency than fusion vaccine. Moreover, intranasal immunization with the nanocage vaccine significantly enhances mucosal immune responses with more efficient B-cell memory generation and antibody maturation, as well as more balanced (Th1/Th2) immune responses with increased IFN-γ and IL-17 production, comparing with fusion vaccine. Mice immunized with the nanocage vaccine exhibited enhanced protection against lethal infection compare to the FlaB-tPspA fusion group. Our study thus demonstrates the effectiveness of an all-in-one nanocage mucosal vaccine platform, which guarantees enhanced protection with balanced immune responses.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

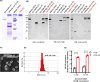

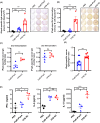
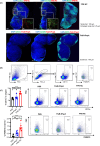
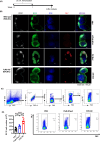


References
-
- Li, M. et al. Mucosal vaccines: Strategies and challenges. Immunol Lett217, 116–125 (2020). - PubMed
-
- Kim, S. A. et al. Protein-based nanocages for vaccine development. J Control Release353, 767–791 (2023). - PubMed
-
- Lee, E. J., Lee, N. K. & Kim, I. S. Bioengineered protein-based nanocage for drug delivery. Adv Drug Deliver Rev106, 157–171 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

