Tm4sf19 inhibition ameliorates inflammation and bone destruction in collagen-induced arthritis by suppressing TLR4-mediated inflammatory signaling and abnormal osteoclast activation
- PMID: 40128226
- PMCID: PMC11933450
- DOI: 10.1038/s41413-025-00419-y
Tm4sf19 inhibition ameliorates inflammation and bone destruction in collagen-induced arthritis by suppressing TLR4-mediated inflammatory signaling and abnormal osteoclast activation
Abstract
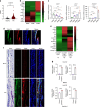
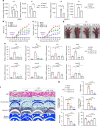
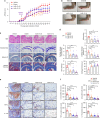
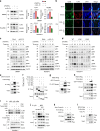
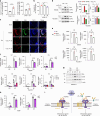
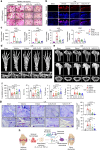
Rheumatoid arthritis (RA) is an autoimmune disease characterized by inflammation and abnormal osteoclast activation, leading to bone destruction. We previously demonstrated that the large extracellular loop (LEL) of Tm4sf19 is important for its function in osteoclast differentiation, and LEL-Fc, a competitive inhibitor of Tm4sf19, effectively suppresses osteoclast multinucleation and prevent bone loss associated with osteoporosis. This study aimed to investigate the role of Tm4sf19 in RA, an inflammatory and abnormal osteoclast disease, using a mouse model of collagen-induced arthritis (CIA). Tm4sf19 expression was observed in macrophages and osteoclasts within the inflamed synovium, and Tm4sf19 expression was increased together with inflammatory genes in the joint bones of CIA-induced mice compared with the sham control group. Inhibition of Tm4sf19 by LEL-Fc demonstrated both preventive and therapeutic effects in a CIA mouse model, reducing the CIA score, swelling, inflammation, cartilage damage, and bone damage. Knockout of Tm4sf19 gene or inhibition of Tm4sf19 activity by LEL-Fc suppressed LPS/IFN-γ-induced TLR4-mediated inflammatory signaling in macrophages. LEL-Fc disrupted not only the interaction between Tm4sf19 and TLR4/MD2, but also the interaction between TLR4 and MD2. μCT analysis showed that LEL-Fc treatment significantly reduced joint bone destruction and bone loss caused by hyperactivated osteoclasts in CIA mice. Taken together, these findings suggest that LEL-Fc may be a potential treatment for RA and RA-induced osteoporosis by simultaneously targeting joint inflammation and bone destruction caused by abnormal osteoclast activation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.J.K. is the founder of Medpacto and serves on its Board of Directors. K.Y., M.W.K., H.J.K., M.J.L., J. B. B., N. P., D.W.K., and S.J.K. are employees of Medpacto. The GILO Foundation has received research support from Medpacto. The other authors declare no competing interests.
Figures
References
-
- Komatsu, N. & Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis - immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol.18, 415–429 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

