Induction of a transcriptional adaptation response by RNA destabilization events
- PMID: 40128410
- PMCID: PMC12069562
- DOI: 10.1038/s44319-025-00427-3
Induction of a transcriptional adaptation response by RNA destabilization events
Abstract
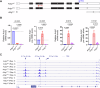
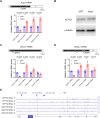
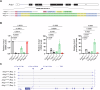
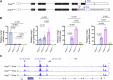
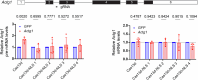
Transcriptional adaptation (TA) is a cellular process whereby mRNA-destabilizing mutations are associated with the transcriptional upregulation of so-called adapting genes. The nature of the TA-triggering factor(s) remains unclear, namely whether an mRNA-borne premature termination codon or the subsequent mRNA decay process, and/or its products, elicits TA. Here, working with mouse Actg1, we first establish two types of perturbations that lead to mRNA destabilization: Cas9-induced mutations predicted to lead to mutant mRNA decay, and Cas13d-mediated mRNA cleavage. We find that both types of perturbations are effective in degrading Actg1 mRNA, and that they both upregulate Actg2. Notably, increased chromatin accessibility at the Actg2 locus was observed only in the Cas9-induced mutant cells but not in the Cas13d-targeted cells, suggesting that chromatin remodeling is not required for Actg2 upregulation. We further show that ribozyme-mediated Actg1 pre-mRNA cleavage also leads to a robust upregulation of Actg2, and that this upregulation is again independent of chromatin remodeling. Together, these data highlight the critical role of RNA destabilization events as a trigger for TA, or at least a TA-like response.
Keywords: CRISPR/Cas13d; CRISPR/Cas9; Self-cleaving Ribozyme; Transcriptional Adaptation; mRNA Decay.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures


Similar articles
-
Transcriptional adaptation upregulates utrophin in Duchenne muscular dystrophy.Nature. 2025 Mar;639(8054):493-502. doi: 10.1038/s41586-024-08539-x. Epub 2025 Feb 12. Nature. 2025. PMID: 39939773 Free PMC article.
-
Genetic compensation triggered by mutant mRNA degradation.Nature. 2019 Apr;568(7751):193-197. doi: 10.1038/s41586-019-1064-z. Epub 2019 Apr 3. Nature. 2019. PMID: 30944477 Free PMC article.
-
Transcriptional adaptation: a mechanism underlying genetic robustness.Development. 2020 Aug 14;147(15):dev186452. doi: 10.1242/dev.186452. Development. 2020. PMID: 32816903 Review.
-
Transcriptional adaptation in Caenorhabditis elegans.Elife. 2020 Jan 17;9:e50014. doi: 10.7554/eLife.50014. Elife. 2020. PMID: 31951195 Free PMC article.
-
A new hypothesis to explain disease dominance.Trends Genet. 2025 Mar;41(3):187-193. doi: 10.1016/j.tig.2024.11.009. Epub 2025 Jan 8. Trends Genet. 2025. PMID: 39788833 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

