Influential drivers of the cost-effectiveness of respiratory syncytial virus vaccination in European older adults: a multi-country analysis
- PMID: 40128710
- PMCID: PMC11934489
- DOI: 10.1186/s12916-025-03970-x
Influential drivers of the cost-effectiveness of respiratory syncytial virus vaccination in European older adults: a multi-country analysis
Abstract
Background: We aimed to identify influential drivers of the cost-effectiveness of older adult respiratory syncytial virus (RSV) vaccination in Denmark, Finland, the Netherlands and Valencia-Spain.
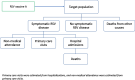
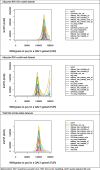
Methods: A static multi-cohort model was parameterised using country- and age-specific hospitalisations using three approaches: (A) the International Classification of Diseases (ICD)-coded hospitalisations, (B) laboratory RSV-confirmed hospitalisations and (C) time-series modelling (TSM). Plausible hypothetical RSV vaccine characteristics were derived from two protein subunit vaccines for adults aged ≥60 years. A full incremental analysis was conducted by comparing three RSV vaccination strategies: (1) in adults aged ≥60 years ("60y+"); (2) in adults aged ≥65 years ("65y+"); (3) in adults aged ≥75 years ("75y+") to "no intervention" and to each other. Both costs and quality-adjusted life-years (QALYs) were discounted at country-specific discount rates and the analysis was conducted from both the healthcare payers' and societal perspectives. Value of information, probabilistic sensitivity and scenario analyses identified influential drivers.
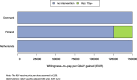
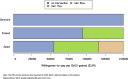
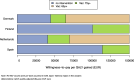
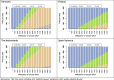
Results: Besides vaccine price, the hospitalisation estimates were most influential: (A) Using adjusted RSV-ICD-coded hospitalisations at a vaccine price of €150 per dose, no intervention was cost-effective up to willingness-to-pay (WTP) values of €150,000 per QALY gained in Denmark and the Netherlands, and up to €124,000 per QALY gained in Finland. (B) Using the adjusted RSV-confirmed dataset, the findings were consistent in Denmark and comparable in Finland. In Spain-Valencia, the 75y+ strategy became cost-effective at WTP >€55,000. (C) Using TSM-based estimates, the 75y+ strategy was cost-effective at WTP >€45,000, >€101,000, >€41,000 and >€114,000 in Denmark, Finland, the Netherlands and Spain-Valencia, respectively. Sensitivity analyses showed that the (in-hospital) case fatality ratio and the specification of its age dependency were both influential. Duration of protection was found more influential than a variety of plausible waning patterns over the duration of protection.
Conclusions: Data gaps and uncertainties on the RSV-related burden in older adults persist and influence the cost-effectiveness of RSV vaccination. More refined age- and country-specific data on the RSV attributable burden are crucial to aid decision making.
Keywords: Ageing population; Cost-effectiveness analysis; Cost-utility analysis; Economic evaluation; Policy; RSV; Respiratory; Uncertainty; Vaccination.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: Outside the submitted work, PB declares funding received by his institute from Merck for research on varicella-zoster and Pfizer for research on pneumococcus vaccine, XL declares funding received by her institute from Icosavax, but they have not received any personal fees or other personal benefits. CKJ reports a research grant from Nordsjællands Hospital, travel grants from the University of Copenhagen, William Demants Fond in Denmark, and the European Society of Clinical Virology and expert consultation fees from Sanofi, outside of the submitted work. Outside the submitted work, HN declares consulting fees received from WHO, Pfizer, Bill and Medlinda Bill and Melinda Gates Foundation and Sanofi, honoraria for lectures/presentations from Astra Zeneca, GSK and Pfizer paid to his institution, travel grants from Pfizer and Sanofi, and participation on a Data Safety Monitoring Board or Advisory Board of GSK, Sanofi, Merck, Icosavax, Pfizer (paid to his institution), as well as WHO and ResViNET (unpaid). AUF declares grants/contracts from Moderna and VAC4EU paid to her institution, outside submitted work. AOS and JDD declares grants/contracts from Moderna, MSD and GSK paid to their institution, outside submitted work. AOS also declares consultancy fees from GSK and Moderna, outside submitted work. JDD declares consultancy fees and honoraria from GSK, Pfizer and MSD, and travel grants from Sanofi and GSK, outside the submitted work. HS declares attending Adult Immunization Board meetings twice a year, the meetings (and travel) are funded by an unrestricted grant from Vaccines Europe, outside submitted work. LW declares grants received from Research Foundation Flanders (FWO), Antwerp University Research Council and Horizon Europe 2.1 – Health paid to his institute, outside the submitted work. MJ declares research grants from NIHR, RCUK, BMGF, WHO, Gavi, Wellcome Trust, European Commission, InnoHK, TFGH, CDC, Gates Foundation paid to his institution, outside the submitted work. Other authors declared no competing interests.
Figures
References
-
- Li Y, Wang X, Broberg EK, Campbell H, Nair H. European RSV Surveillance Network. Seasonality of respiratory syncytial virus and its association with meteorological factors in 13 European countries, week 40 2010 to week 39 2019. Euro Surveill. 2022;27(16):2100619. 10.2807/1560-7917.ES.2022.27.16.2100619. - PMC - PubMed
-
- Shi T, Vennard S, Jasiewicz F, Brogden R, Nair H. Disease Burden Estimates of Respiratory Syncytial Virus related Acute Respiratory Infections in Adults With Comorbidity: A Systematic Review and Meta-Analysis. J Infect Dis. 2022;226(Suppl 1):S17–s21. - PubMed
-
- Wilson E, Goswami J, Baqui AH, Doreski PA, Perez-Marc G, Zaman K, Monroy J, Duncan CJA, Ujiie M, Ramet M, et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N Engl J Med. 2023;389(24):2233–44. - PubMed
-
- Walsh EE, Pérez Marc G, Zareba AM, Falsey AR, Jiang Q, Patton M, Polack FP, Llapur C, Doreski PA, Ilangovan K, et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N Engl J Med. 2023;388(16):1465–77. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

