Simultaneous Monitoring of Tyrosinase and ATP in Thick Brain Tissues Using a Single Two-Photon Fluorescent Probe
- PMID: 40129186
- PMCID: PMC12097068
- DOI: 10.1002/advs.202413220
Simultaneous Monitoring of Tyrosinase and ATP in Thick Brain Tissues Using a Single Two-Photon Fluorescent Probe
Abstract
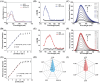
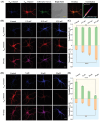
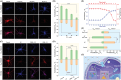
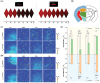
Cellular redox homeostasis and energy metabolism in the central nervous system are associated with neurodegenerative diseases. However, their real-time and concurrent monitoring in thick tissues remains challenging. Herein, a single dual-emission two-photon fluorescent probe (named DST) is designed for the simultaneous tracking of tyrosinase (TYR) and adenosine triphosphate (ATP), thereby enabling the real-time monitoring of both neurocellular redox homeostasis and energy metabolism in brain tissue. The developed DST probe exhibits excellent sensitivity and selectivity toward TYR and ATP, with distinctive responses in the blue and red fluorescence channels being observed without spectra crosstalk. Using this probe, the correlation and regulatory mechanism between TYR and ATP during oxidative stress are uncovered. Additionally, the two-photon nature of this probe allows alterations in the TYR and ATP levels to be monitored across different brain regions in an Alzheimer's disease (AD) mouse model. Notably, a significant decrease in ATP levels is revealed within the somatosensory cortex (S1BF) and caudate putamen brain regions of an AD mouse, alongside an increase in TYR levels within the S1BF and laterodorsal thalamic nucleus brain regions. These findings indicate the potential of applying the spatially resolved regulation of neurocellular redox homeostasis and energy metabolism to treat neurodegenerative diseases.
Keywords: ATP; alzheimer's disease; brain tissues; two‐photon imaging; tyrosinase.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Simultaneous Sensing of H2S and ATP with a Two-Photon Fluorescent Probe in Alzheimer's Disease: toward Understanding Why H2S Regulates Glutamate-Induced ATP Dysregulation.Anal Chem. 2022 Aug 23;94(33):11573-11581. doi: 10.1021/acs.analchem.2c01850. Epub 2022 Aug 9. Anal Chem. 2022. PMID: 35943780
-
A colorimetric and near -infrared ratiometric fluorescent probe for the determination of endogenous tyrosinase activity based on cyanine aggregation.Analyst. 2019 Sep 21;144(18):5472-5478. doi: 10.1039/c9an01045a. Epub 2019 Aug 6. Analyst. 2019. PMID: 31384852
-
Highly selective two-photon fluorescent off-on probes for imaging tyrosinase activity in living cells and tissues.Chem Commun (Camb). 2021 Jul 13;57(56):6911-6914. doi: 10.1039/d1cc02374h. Chem Commun (Camb). 2021. PMID: 34152336
-
The Triangle of Death in Alzheimer's Disease Brain: The Aberrant Cross-Talk Among Energy Metabolism, Mammalian Target of Rapamycin Signaling, and Protein Homeostasis Revealed by Redox Proteomics.Antioxid Redox Signal. 2017 Mar 10;26(8):364-387. doi: 10.1089/ars.2016.6759. Epub 2016 Oct 20. Antioxid Redox Signal. 2017. PMID: 27626216 Review.
-
Redox imbalance and metabolic defects in the context of Alzheimer disease.FEBS Lett. 2024 Sep;598(17):2047-2066. doi: 10.1002/1873-3468.14840. Epub 2024 Mar 12. FEBS Lett. 2024. PMID: 38472147 Review.
References
-
- a) Kang Z., Jiang J., Tu Q., Liu S., Zhang Y., Wang D.‐E., Wang J., Yuan M.‐S., J. Am. Chem. Soc. 2023, 145, 507; - PubMed
- b) Brewster J. T. II, Dell'Acqua S., Thach D. Q., Sessler J. L., ACS Chem. Neurosci. 2019, 10, 155; - PubMed
- c) Liesz A., Science 2019, 365, 223; - PubMed
- d) Zhang L., Peng S., Sun J., Yao J., Kang J., Hu Y., Fang J., Chem. Sci. 2017, 8, 2966. - PMC - PubMed
-
- a) Fujieda N., Umakoshi K., Ochi Y., Nishikawa Y., Yanagisawa S., Kubo M., Kurisu G., Itoh S., Angew. Chem., Int. Ed. 2020, 59, 13385; - PubMed
- b) Zhang T., Li Y., Guo J., Sun W., Lv Y., Adv. Healthcare Mater. 2024, 13, 2303615; - PubMed
- c) Kampatsikas I., Bijelic A., Pretzler M., Rompel A., Angew. Chem., Int. Ed. 2019, 58, 7475. - PMC - PubMed
-
- a) Wu Z., He K., Chen Y., Li H., Pan S., Li B., Liu T., Xi F., Deng F., Wang H., Du J., Jing M., Li Y., Neuron 2022, 110, 770 ; - PubMed
- b) Liu J., Zhang W., Wang X., Ding Q., Wu C., Zhang W., Wu L., James T. D., Li P., Tang B., J. Am. Chem. Soc. 2023, 145, 19662; - PMC - PubMed
- c) Xu X., Fei J., Xu Y., Li G., Dong W., Xue H., Li J., Angew. Chem., Int. Ed. 2021, 60, 7617. - PubMed
-
- a) Yan H., Wang Y., Huo F., Yin C., J. Am. Chem. Soc. 2023, 145, 3229; - PubMed
- b) Brady M., Shchepetkina V. I., González‐Recio I., Martínez‐Chantar M. L., Buccella D., J. Am. Chem. Soc. 2023, 145, 21841; - PMC - PubMed
- c) Fang H., Chen Y., Jiang Z., He W., Guo Z., Acc. Chem. Res. 2023, 56, 258. - PubMed
-
- a) Tamima U., Sarkar S., Islam M. R., Shil A., Kim K. H., Reo Y. J., Jun Y. W., Banna H., Lee S., Ahn K. H., Angew. Chem., Int. Ed. 2023, 62, e202300580; - PubMed
- b) Yang S., Jiang J., Zhou A., Zhou Y., Ye W., Cao D.‐S., Yang R., Anal. Chem. 2020, 92, 7194; - PubMed
- c) Morozov B. S., Gargiulo F., Ghule S., Lee D. J., Hampel F., Kim H. M., Kataev E. A., J. Am. Chem. Soc. 2024, 146, 7105; - PubMed
- d) Sun P., Chen H.‐C., Lu S., Hai J., Guo W., Jing Y.‐H., Wang B., Anal. Chem. 2022, 94, 11573; - PubMed
- e) Cui Y., Park S. J., Wu X., Wang R., Qi S., Kim H. M., Yoon J., Chem. Commun. 2021, 57, 6911. - PubMed
MeSH terms
Substances
Grants and funding
- 22074100/National Natural Science Foundation of China
- 2022YFF1103000/National Key R&D Program of China
- 2023AY40021/Special Project for Young Scientific and Technological Talents of Jiaxing City
- 2308085MB57/Anhui Provincial Natural Science Foundation
- 2022e07020060/Key Research and Development Program of Anhui Province
LinkOut - more resources
Full Text Sources
