Levodopa-Entacapone-Carbidopa Intrajejunal Infusion in Advanced Parkinson's Disease - Interim Analysis of the ELEGANCE Study
- PMID: 40129359
- PMCID: PMC12371452
- DOI: 10.1002/mdc3.70046
Levodopa-Entacapone-Carbidopa Intrajejunal Infusion in Advanced Parkinson's Disease - Interim Analysis of the ELEGANCE Study
Abstract
Background: Levodopa-entacapone-carbidopa intestinal gel (LECIG) was introduced in 2018 as a device-aided therapy for advanced Parkinson's disease (PD).
Objectives: The ELEGANCE study (NCT05043103) is gathering real-world data on long-term efficacy, safety and patient-reported outcomes with LECIG from 13 European countries. This article reports data from the planned interim analysis.
Methods: The study enrolled patients prescribed LECIG as part of routine clinical care. We evaluated patients at V1 before starting LECIG treatment (in seven patients V1 data were obtained retrospectively), and thereafter at V2 (3-6 months) or V3 (6-12 months).
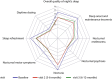
Results: This analysis includes 167 patients from 37 centers. Three patients from this analysis set (1.8%) discontinued the study. Mean (±SD) daily OFF-time hours (MDS-UPDRS IV item 4.3) were substantially reduced by 3.47 ± 3.56 h at V2 (baseline: 5.15 ± 3.05; P < 0.0001). Similarly, MDS-UPDRS part IV total scores were reduced by 4.24 ± 4.08 at V2 (baseline: 10.77 ± 3.83); (P = 0.0001) and MDS-UPDRS part II scores by 3.63 ± 7.76 at V2 (baseline: 20.65 ± 8.17; P = 0.0004). PDSS-2 total scores were sustainably improved (reduction of 7.38 ± 10.72 at V2 [baseline: 25.21 ± 10.62]; P < 0.0001), as was the PDQ-8 summary index score indicating an improvement in quality of life (QoL) (reduction of 13.3 ± 19.05 at V2 [baseline: 46.34 ± 20.09]; P < 0.0001). For all parameters improvements were maintained at V3. Patient-reported satisfaction with the LECIG pump was high. Most adverse events were related to the procedure or the device.
Conclusions: Routine use of LECIG for up to 12 months provided sustained control of motor symptoms, and was well tolerated with a positive impact on QoL and high patient satisfaction.
Keywords: advanced Parkinson's disease; carbidopa intestinal gel; clinical practice; entacapone; levodopa.
© 2025 The Author(s). Movement Disorders Clinical Practice published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Conflict of interest statement
Figures

References
-
- de Bie RMA, Clarke CE, Espay AJ, Fox SH, Lang AE. Initiation of pharmacological therapy in Parkinson's disease: when, why, and how. Lancet Neurol 2020;19(5):452–461. - PubMed
-
- Weiss D, Volkmann J, Fasano A, Kuhn A, Krack P, Deuschl G. Changing gears ‐ DBS for dopaminergic desensitization in Parkinson's disease? Ann Neurol 2021;90(5):699–710. - PubMed
-
- Tanner CM. Exploring the clinical burden of OFF periods in Parkinson disease. Am J Manag Care 2020;26(12 Suppl):S255–S264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

