Pilot study of a novel lumen-apposing metal stent for endoscopic ultrasound-guided procedures in porcine models
- PMID: 40129530
- PMCID: PMC11930856
- DOI: 10.1002/deo2.70084
Pilot study of a novel lumen-apposing metal stent for endoscopic ultrasound-guided procedures in porcine models
Abstract
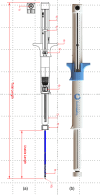
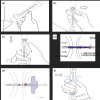
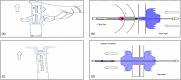
Lumen-apposing metal stents have expanded the therapeutic potential of interventional endoscopic ultrasound (EUS). The Hot-Spaxus (Taewoong Medical Co., Ltd.), the second most commonly utilized lumen-apposing metal stent, requires two operators for its release which has been considered a limitation compared to other lumen-apposing metal stents. We aimed to test the feasibility and the technical success of a newly available version of the Hot-Spaxus stent equipped with an innovative handle delivery system for EUS-guided interventional procedures. We conducted a pilot study using porcine models. The novel Hot-Spaxus 2 was tested by performing four EUS-guided procedures including four EUS-guided gallbladder drainage and 12 EUS-guided gastrojejunostomy) procedures. Technical success was reported in 100% of cases. The mean procedure time for EUS-guided gatrojejunostomyJ and EUS-guided gallbladder drainage was 23.85 min (standard deviation 3.41) and 16.15 min (standard deviation 2.72), respectively. The distal and proximal flanges were safely released by the endosonographer without any complications. No adverse events were reported. In conclusion, the novel Hot-Spaxus 2 stent may represent an improvement compared to the prior Spaxus model. Unlike its predecessor, this newly designed stent eliminates the need for two endoscopists and can be deployed by a single operator. Further human studies are necessary to validate its clinical effectiveness.
Keywords: EUS‐GBD; EUS‐GE; Hot‐Spaxus; endoscopic ultrasound; lumen‐apposing metal stents.
© 2025 The Author(s). DEN Open published by John Wiley & Sons Australia, Ltd on behalf of Japan Gastroenterological Endoscopy Society.
Conflict of interest statement
Benedetto Mangiavillano is a consultant for Taewoong; Alberto Larghi is a consultant for Boston Scientific; Alberto Larghi is a consultant for Boston Scientific, Fujifilm, and Medtronic. The other authors declare no conflict of interest.
Figures
References
-
- Debourdeau A, Daniel J, Caillo L et al. Effectiveness of endoscopic ultrasound (EUS)‐guided choledochoduodenostomy vs. EUS‐guided gallbladder drainage for jaundice in patients with malignant distal biliary obstruction after failed endoscopic retrograde cholangiopancreatography: Retrospective, multicenter study (GALLBLADEUS Study). Dig Endosc 2025; 37: 103–14. - PMC - PubMed
-
- Fugazza A, Fabbri C, Di Mitri R et al. EUS‐guided choledochoduodenostomy for malignant distal biliary obstruction after failed ERCP: A retrospective nationwide analysis. Gastrointest Endosc 2022; 95: 896–904.e1. - PubMed
-
- Mangiavillano B, Moon JH, Facciorusso A et al. Endoscopic ultrasound‐guided gallbladder drainage as a first approach for jaundice palliation in unresectable malignant distal biliary obstruction: Prospective study. Dig Endosc 2024; 36: 351–8. - PubMed
-
- van der Merwe SW, van Wanrooij RLJ, Bronswijk M et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2022; 54: 185–205. - PubMed
LinkOut - more resources
Full Text Sources
