Real-world effectiveness of CDK4/6i in first-line treatment of HR+/HER2- advanced/metastatic breast cancer: updated systematic review
- PMID: 40129925
- PMCID: PMC11931418
- DOI: 10.3389/fonc.2025.1530391
Real-world effectiveness of CDK4/6i in first-line treatment of HR+/HER2- advanced/metastatic breast cancer: updated systematic review
Abstract
Aim: Since 2021, additional real-world evidence (RWE) has emerged on the effectiveness of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) as first-line treatment of HR-positive/HER2-negative (HR+/HER2-) advanced/metastatic breast cancer (A/MBC), necessitating this updated review.
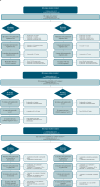
Methods: MEDLINE®, Embase®, and Cochrane Databases (07/06/2019-01/09/2024), and key congresses (2020-2024) were searched. Studies reporting first-line CDK4/6i use, over 100 participants, and progression-free survival (PFS) and/or overall survival (OS) data were included.
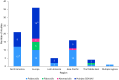
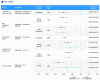
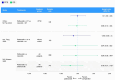
Results: This update included 82 unique studies, 42.7% for palbociclib, 7.3% for ribociclib, and 3.7% for abemaciclib; 46.3% assessed multiple CDK4/6i. In studies including multiple CDK4/6is, median PFS was 23.4-31.0 months for palbociclib, 19.8-44.0 for ribociclib, and 14.0-39.5 for abemaciclib. When reached, median OS was 38.0-58.0 months, 40.4-52.0 months, and 34.4 months, respectively. These real-world PFS and OS results were within the range of single-arm and CDK4/6i versus endocrine therapy (ET) studies, where CDK4/6i demonstrated greater benefits than ET alone.
Conclusion: First-line CDK4/6i RWE demonstrates significant clinical benefits in HR+/HER2- A/MBC. These data are important to guide clinical decision-making, as they include patients who are not adequately represented in clinical trials. Studies with longer follow-up are needed to assess long-term benefits of all three CDK4/6i therapies in HR+/HER2- A/MBC.
Keywords: CDK4/6 inhibitors; HR+/HER2−; breast; metastasis; quality assessment; real-world evidence; systematic literature review.
Copyright © 2025 Harbeck, Brufsky, Rose, Korytowsky, Chen, Tantakoun, Jazexhi, Nguyen, Bartlett, Samjoo and Pluard.
Conflict of interest statement
CR, BK, and CC were employed by the company Pfizer Inc. NH, AB, and TP were consultants for the company Pfizer Inc. MB, KT, DN, EJ, and IS were employees of the company EVERSANA, Canada, which was a paid consultant to Pfizer Inc. in connection with the development of this manuscript.
Figures
References
-
- Ferlay J EM, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, et al. . Global cancer observatory: Cancer today . Available online at: https://gco.iarc.who.int/today (Accessed July 2024).
-
- Health NIo . Cancer stat facts: Female breast cancer . Available online at: https://seer.cancer.gov/statfacts/html/breast.html (Accessed May 2024).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

