Highly efficient in vivo hematopoietic stem cell transduction using an optimized self-complementary adeno-associated virus
- PMID: 40129926
- PMCID: PMC11930595
- DOI: 10.1016/j.omtm.2025.101438
Highly efficient in vivo hematopoietic stem cell transduction using an optimized self-complementary adeno-associated virus
Abstract
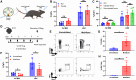
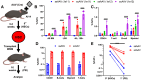
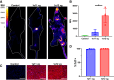
In vivo gene therapy targeting hematopoietic stem cells (HSCs) holds significant therapeutic potential for treating hematological diseases. This study uses adeno-associated virus serotype 6 (AAV6) vectors and Cre recombination to systematically optimize the parameters for effective in vivo HSC transduction. We evaluated various genetic architectures and delivery methods of AAV6, establishing an optimized protocol that achieved functional recombination in more than two-thirds of immunophenotypic HSCs. Our findings highlight that second-strand synthesis is a critical limiting factor for transgene expression in HSCs, leading to significant under-detection of HSC transduction with single-stranded AAV6 vectors. We also demonstrate that HSCs in the bone marrow (BM) are readily accessible to transduction, with neither localized injection nor mobilization of HSCs into the bloodstream, enhancing transduction efficacy. Additionally, we observed a surprising preference for HSC transduction over other BM cells, regardless of the AAV6 delivery route. Together, these findings not only underscore the potential of AAV vectors for in vivo HSC gene therapy but also lay a foundation that can inform the development of both in vivo AAV-based HSC gene therapies and potentially in vivo HSC gene therapies that employ alternative delivery modalities.
Keywords: AAV; HSC; adeno-associated virus; bone marrow transduction; genetic hematological disease; in vivo gene therapy; second-strand synthesis; self-complementary; systemic delivery; targeted integration.
© 2025 The Authors.
Conflict of interest statement
H.N. is a co-founder of and shareholder in Megakaryon, and Century Therapeutics. A.C.W. is a consultant for ImmuneBridge. A.C. discloses financial interests in the following entities working in the rare genetic disease space: Beam Therapeutics, Diantus Therapeutics, Editas Medicines, GV, Inograft Biotherapeutics, Jasper Therapeutics, Kyowa Kirin, Prime Medicine, Rocket Pharmaceuticals, STRM.Bio, Spotlight Therapeutics, and Teiko Bio. However, none of these companies had input into the design, execution, interpretation, or publication of the work in this manuscript.
Figures



References
-
- Wilkinson A.C., Dever D.P., Baik R., Camarena J., Hsu I., Charlesworth C.T., Morita C., Nakauchi H., Porteus M.H. Cas9-AAV6 gene correction of beta-globin in autologous HSCs improves sickle cell disease erythropoiesis in mice. Nat. Commun. 2021;12:686. doi: 10.1038/s41467-021-20909-x. - DOI - PMC - PubMed

