Urinary microbiota changes among NMIBC patients during BCG therapy: comparing BCG responders and non-responders
- PMID: 40129930
- PMCID: PMC11931020
- DOI: 10.3389/fcimb.2025.1479795
Urinary microbiota changes among NMIBC patients during BCG therapy: comparing BCG responders and non-responders
Abstract
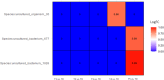
The gold standard for treating high-risk non-muscle-invasive bladder cancer involves the transurethral removal of cancerous tissue followed by BCG immunotherapy. So far, there is no reliable biomarker for predicting BCG efficacy and identifying patients who will or will not respond to BCG treatment. Emerging evidence suggests that urinary microbiota may play a crucial role in BCG efficacy. This study aimed to explore (i) changes in urinary microbiota during the six induction cycles of BCG and (ii) its potential predictive role in determining the outcome of BCG treatment. To this end, catheterized urine samples were collected before each of the six BCG doses and bacterial composition was analyzed using 16S rRNA gene sequencing. Patient inclusion criteria were male gender, no previous history of urothelial cancer, no other malignancies, no active infection, and no antibiotic usage for at least 20 days before the first BCG dose. We observed a significant decrease in biodiversity, measured by the Shannon Index, during the first week of therapy in 10 out of 12 patients (p=0.021). Additionally, differences in microbiota composition before the start of BCG therapy were noted between responders and non-responders to BCG therapy. Non-responders exhibited a 12 times higher abundance of genus Aureispira (p<0.001), and, at the species level, a 27-fold lower abundance of Negativicoccus succinivorans (p<0.001). Throughout the treatment, the abundance of the genus Aureispira decreased, showing an eightfold reduction by the end of therapy among non-responders (p<0.001). Our findings suggest that urinary microbiota plays an active role before and during the course of BCG therapy. However, this is a preliminary study, and further research involving larger patient cohorts is needed.
Keywords: BCG; immunotherapy; non-muscle invasive bladder cancer; response to therapy; urinary microbiome.
Copyright © 2025 Boban, Milić Roje, Knezović, Jerončić, Šošić, Šitum and Terzić.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

