Prospective Evaluation of the Safety and Compression Performance of Novel Compression Denim Jeans in Healthy Volunteers and Patients With Lymphedema
- PMID: 40129954
- PMCID: PMC11930548
- DOI: 10.7759/cureus.80971
Prospective Evaluation of the Safety and Compression Performance of Novel Compression Denim Jeans in Healthy Volunteers and Patients With Lymphedema
Abstract
Objectives: The treatment of lower-extremity lymphedema, whether congenital or acquired, remains challenging. Long-term management aimed at reducing complications and maximizing quality of life is essential. Compression stockings are crucial in this management; however, their application is limited by patient experience (ease of wear, texture, breathability, and appearance). This highlights the need to evaluate alternative compression garments that maintain therapeutic efficacy while improving patient adherence.
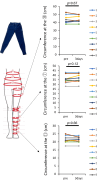
Methods: We developed a novel compression denim product (Flow plus Jeans®) using advanced sewing technology. Its baseline performance (compression ability) was evaluated by measuring pressure gradients at three points (ankle, calf, and thigh) using a mannequin-based compression testing system and compared with those of existing stockings. Thereafter, a safety assessment was conducted on healthy volunteers to evaluate potential adverse effects, including changes in lower limb circumference, signs of deep vein thrombosis (DVT) via ultrasound, and skin complications. A clinical trial in patients with lymphedema was then performed to compare its efficacy with that of conventional compression stockings.
Results: Baseline performance testing with a mannequin revealed that Flow plus Jeans demonstrated compression levels and pressure gradients at three calf points comparable to those of standard compression stockings. A safety study involving nine healthy volunteers confirmed that Flow plus Jeans caused no significant changes in lower-limb circumferences after three days of wear, with no cases of DVT or skin complications. In a subsequent clinical trial involving nine female patients with lymphedema, the jeans showed non-inferiority to existing stockings concerning lower-limb circumference measurements at six points (pre-use vs. six months post-use), with patient-reported experiences assessed via questionnaires. Notably, patients reported enhanced satisfaction regarding the jeans' fashionability, which could serve as an incentive for long-term adherence.
Conclusion: Our findings suggest that Flow plus Jeans represent a promising novel option for the long-term management of lymphedema, offering an alternative that balances medical efficiency with improved patient satisfaction and demonstrates safety in healthy individuals.
Keywords: compression garments; denim jeans; long-term management; lower-extremity lymphedema; quality of life.
Copyright © 2025, Ousaka et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Okayama University Hospital Ethics Committee issued approval 2106-045. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Susumu Oozawa and Satoe Kirino declare(s) non-financial support from Stork Visit Co., Ltd. Stork Visit Co., Ltd. is a university-originated venture from Okayama University, founded by authors Susumu Oozawa and Satoe Kirino. Flow plus Jeans® is a registered trademark of this company. Intellectual property info: The authors declare that they have developed the medical device, Flow Plus Jeans®, used in this study. A patent related to the device has been obtained by Susumu Oozawa, and the intellectual property rights are held by Susumu Oozawa or Okayama University. However, the study was conducted independently, and the results presented are not influenced by any financial or commercial interests. Other relationships: Takumi Takahashi, Akihiro Matsuoka, Shintaro Yamada are employees of the jeans development company. They were involved in the development of Flow plus Jeans®, including the prototypes used in the preliminary study. Additionally, they contributed to the mannequin-based compression pressure measurement experiments.
Figures












Similar articles
-
Prevention of post thrombotic syndrome with Pycnogenol® in a twelve month study.Panminerva Med. 2011 Sep;53(3 Suppl 1):21-7. Panminerva Med. 2011. PMID: 22108473 Clinical Trial.
-
A compression device versus compression stockings in long-term therapy of lower limb primary lymphoedema after liposuction.J Wound Care. 2020 Jan 2;29(1):28-35. doi: 10.12968/jowc.2020.29.1.28. J Wound Care. 2020. PMID: 31930941
-
Endovascular radiofrequency ablation for varicose veins: an evidence-based analysis.Ont Health Technol Assess Ser. 2011;11(1):1-93. Epub 2011 Feb 1. Ont Health Technol Assess Ser. 2011. PMID: 23074413 Free PMC article.
-
Compression Stockings for the Prevention of Venous Leg Ulcer Recurrence: A Health Technology Assessment.Ont Health Technol Assess Ser. 2019 Feb 19;19(2):1-86. eCollection 2019. Ont Health Technol Assess Ser. 2019. PMID: 30828407 Free PMC article.
-
Compression therapy for treating post-thrombotic syndrome.Cochrane Database Syst Rev. 2019 Sep 18;9(9):CD004177. doi: 10.1002/14651858.CD004177.pub2. Cochrane Database Syst Rev. 2019. PMID: 31531971 Free PMC article.
References
-
- Overview of lymphedema for physicians and other clinicians: a review of fundamental concepts. Manrique OJ, Bustos SS, Ciudad P, et al. Mayo Clin Proc. 2022;97:1920–1935. - PubMed
-
- Long-term management of breast cancer-related lymphedema after intensive decongestive physiotherapy. Vignes S, Porcher R, Arrault M, Dupuy A. Breast Cancer Res Treat. 2007;101:285–290. - PubMed
-
- Lower-limb lymphedema and vulval cancer: feasibility of prophylactic compression garments and validation of leg volume measurement. Sawan S, Mugnai R, Lopes Ade B, Hughes A, Edmondson RJ. Int J Gynecol Cancer. 2009;19:1649–1654. - PubMed
-
- Therapy modalities to reduce lymphoedema in female breast cancer patients: a systematic review and meta-analysis. Rogan S, Taeymans J, Luginbuehl H, Aebi M, Mahnig S, Gebruers N. Breast Cancer Res Treat. 2016;159:1–14. - PubMed
-
- A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. Lasinski BB, McKillip Thrift K, Squire D, et al. PM R. 2012;4:580–601. - PubMed
LinkOut - more resources
Full Text Sources
