Checkpoint kinase 1 in colorectal cancer: Upregulation of expression and promotion of cell proliferation
- PMID: 40130044
- PMCID: PMC11866088
- DOI: 10.5306/wjco.v16.i3.101725
Checkpoint kinase 1 in colorectal cancer: Upregulation of expression and promotion of cell proliferation
Abstract
Background: Colorectal cancer (CRC) is a prevalent malignant tumor characterized by a high mortality rate, with significant challenges persisting in the identification and management of its metastatic stage. The role of checkpoint kinase 1 (CHEK1), a cell cycle checkpoint kinase, in CRC has not been fully clarified. We hypothesize that the upregulation of CHEK1 may enhance the proliferation of CRC cells, indicating its potential as a novel therapeutic target for CRC therapy.
Aim: To investigate the expression and function of CHEK1 in CRC, this study utilizes single-cell RNA sequencing and tissue microarray data.
Methods: Single-cell RNA sequencing technology was employed to analyze CRC cells from the GSE144735 dataset, and immunohistochemistry was conducted to confirm the expression of CHEK1 in CRC and adjacent tissues. We also integrated mRNA expression data from multiple public databases to assess global CHEK1 expression in CRC. Molecular docking experiments were performed to explore the interaction between CHEK1 and the potential drug nitidine chloride (NC), as well as to investigate the influence of CHEK1 on CRC cell proliferation.
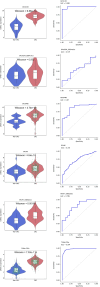
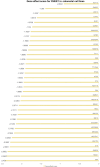
Results: We found comparatively elevated CHEK1 expression in the malignant epithelial cells of CRC, with marked upregulation of its mRNA levels in CRC tissues. Immunohistochemical analysis further confirmed the high expression of CHEK1 in CRC tissues, and the receiver operating characteristic curve demonstrated high accuracy (area under the curve = 0.964) for CHEK1 as a biomarker. Analysis of global datasets indicated a statistically significant overexpression of CHEK1 in CRC (standard mean difference = 1.81, P < 0.01), with summary receiver operating characteristic analysis yielding sensitivity and specificity values of 0.83 and 0.88, respectively. Molecular docking studies indicated that NC specifically targeted CHEK1, while clustered regularly interspaced short palindromic repeats knockout experiments demonstrated that CHEK1 promoted CRC cell proliferation.
Conclusion: Upregulation of CHEK1 promotes CRC cell proliferation. However, the dataset's diversity is limited, requiring further investigation into its specific mechanisms.
Keywords: Checkpoint kinase 1; Colorectal cancer; Immunohistochemistry; Molecular docking; Single-cell sequencing.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures








References
-
- Klimeck L, Heisser T, Hoffmeister M, Brenner H. Colorectal cancer: A health and economic problem. Best Pract Res Clin Gastroenterol. 2023;66:101839. - PubMed
-
- Andrei P, Battuello P, Grasso G, Rovera E, Tesio N, Bardelli A. Integrated approaches for precision oncology in colorectal cancer: The more you know, the better. Semin Cancer Biol. 2022;84:199–213. - PubMed
-
- Kong WS, Li JJ, Deng YQ, Ju HQ, Xu RH. Immunomodulatory molecules in colorectal cancer liver metastasis. Cancer Lett. 2024;598:217113. - PubMed
-
- Wang Y, Wu S, Song Z, Yang Y, Li Y, Li J. Unveiling the pathological functions of SOCS in colorectal cancer: Current concepts and future perspectives. Pathol Res Pract. 2024;262:155564. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

