From adhesion to biofilms formation and resilience: Exploring the impact of silver nanoparticles-based biomaterials on Pseudomonas aeruginosa
- PMID: 40130065
- PMCID: PMC11930599
- DOI: 10.1016/j.bioflm.2025.100267
From adhesion to biofilms formation and resilience: Exploring the impact of silver nanoparticles-based biomaterials on Pseudomonas aeruginosa
Abstract
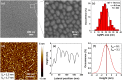
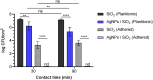
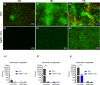
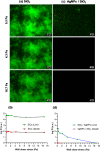
Colonization of medical devices by microorganisms, often progressing to the formation of resilient biofilms, presents a common clinical issue. To address this challenge, there is growing interest in developing novel biomaterials with antimicrobial/antibiofilm properties as a promising preventive measure. This study explores nanocomposite biomaterials based on silver nanoparticles (AgNPs) deposited on thin silica (SiO2) layers for their potential effect on the adhesion, detachment, viability and biofilm formation of the opportunistic Pseudomonas aeruginosa. The AgNPs-based biointerface effect on biofilm development is investigated on the PAO1-Tn7-gfp strain by combining experiments under static and dynamic conditions. For the latter, a shear-stress flow chamber is used to mimic conditions encountered around certain medical devices. The findings reveal a rapid bactericidal effect of the AgNPs, noticeable within 30 min of exposure. Moreover, a delay in surface colonization is observed with a thin and unstructured biofilm, even after 72h of dynamic culture. A considerable fragility and sensitivity to hydrodynamic stresses is noticed for this loosely attached bacterial monolayer when compared with the thick and resilient biofilm formed on SiO2 surface. This study underlines the potential of AgNPs-based biomaterials in the conception of novel antimicrobial/antibiofilm surfaces with controlled release of the biocidal agent.
Keywords: Antiadhesion; Antibiofilm; Antimicrobial; Biointerfaces; Pseudomonas aeruginosa; Silver nanoparticle-based biomaterials; plasma (gas discharge) process.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kremena Makasheva reports financial support was provided by 10.13039/501100001665French National Research Agency. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
LinkOut - more resources
Full Text Sources

