Engineering a stem cell-embedded bilayer hydrogel with biomimetic collagen mineralization for tendon-bone interface healing
- PMID: 40130078
- PMCID: PMC11931223
- DOI: 10.1016/j.bioactmat.2025.03.001
Engineering a stem cell-embedded bilayer hydrogel with biomimetic collagen mineralization for tendon-bone interface healing
Abstract
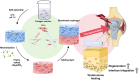
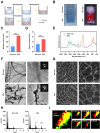
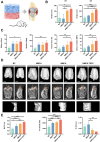
The tendon-bone interface effectively transfers mechanical stress for movement, yet its regeneration presents significant clinical challenges due to its hierarchical structure and composition. Biomimetic strategies that replicate the distinctive characteristics have demonstrated potential for enhancing the healing process. However, there remains a challenge in developing a composite that replicates the nanostructure of the tendon-bone interface and embeds living cells. Here, we engineered a nanoscale biomimetic bilayer hydrogel embedded with tendon stem cells for tendon-bone interface healing. Specifically, the biomimetic hydrogel incorporates intra- and extrafibrillar mineralized collagen fibrils as well as non-mineralized collagen fibrils resembling the tendon-bone interface at the nanoscale. Furthermore, biomimetic mineralization with the presence of cells realizes living tendon-bone-like tissue constructs. In the in vivo patella-patellar tendon-interface injury model, the tendon stem cell-laden biomimetic hydrogel promoted tendon-bone interface regeneration, demonstrated by increased fibrocartilage formation, improved motor function, and enhanced biomechanical outcomes. This study highlights the potential of the stem cell-laden biomimetic hydrogel as an effective strategy for tendon-bone interface regeneration, offering a novel approach to engineering complex tissue interfaces.
Keywords: Biomimetic mineralization; Hydrogel; Tendon stem cell; Tendon-bone interface.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Golman M., Abraham A.C., Kurtaliaj I., Marshall B.P., Hu Y.J., Schwartz A.G., Guo X.E., Birman V., Thurner P.J., Genin G.M., Thomopoulos S. Toughening mechanisms for the attachment of architectured materials: the mechanics of the tendon enthesis. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abi5584. eabi5584. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

