Safe Sedation and Analgesia Management With Remifentanil in Pediatric ICU Patients: A Report of Two Cases
- PMID: 40130113
- PMCID: PMC11931403
- DOI: 10.7759/cureus.79435
Safe Sedation and Analgesia Management With Remifentanil in Pediatric ICU Patients: A Report of Two Cases
Abstract
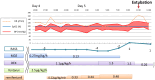
Although remifentanil (RF) is not approved for use in the ICU for children, its short half-life and titratability may provide advantages in children requiring high doses of sedatives and analgesics during prolonged mechanical ventilation. This report presents two pediatric cases in which RF was administered for sedation and analgesia, enabling successful extubation without adverse effects. In Case 1, a four-year-old girl with multiple atypical teratoid rhabdoid tumors required mechanical ventilation due to impaired consciousness. RF was initiated at 0.22 μg/kg/min and titrated up to 0.48 μg/kg/min on the day before extubation, allowing the reduction of other sedatives. She received RF for a total of 16 hours (5.66 mg) before it was discontinued, and extubation was successfully performed 70 minutes later. In Case 2, a three-year-old girl with a severe neck abscess required prolonged intubation following surgical drainage. RF was started at 0.1 μg/kg/min and increased to 0.2 μg/kg/min on the day before extubation, facilitating the tapering of other sedatives. She received RF for a total of 23 hours (4.4 mg) before discontinuation, and extubation was successfully performed 25 minutes later. Throughout RF administration, hemodynamic stability was maintained, with no occurrences of hypotension, bradycardia, desaturation, hepatic or renal dysfunction, muscle rigidity, or hyperalgesia. No additional catecholamines were required, and no signs of withdrawal symptoms were observed. These findings suggest that RF may be a safe and effective option for sedation and analgesia in pediatric patients undergoing prolonged mechanical ventilation.
Keywords: analgesia; intensive care unit; pediatric; remifentanil; sedation.
Copyright © 2025, Tamaki et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Unapproved New Drug Evaluation Committee issued approval TF23003. The Unapproved New Drug Evaluation Committee approved the off-label use of remifentanil for pediatric patients on March 20, 2023. The targeted patients are pediatric patients undergoing mechanical ventilation in intensive care settings. Furthermore, this study has been approved by our hospital's Bioethics Review Committee (approval number: 2024-0305). Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


Similar articles
-
[Remifentanil for analgesia and sedation in mechanically ventilated patients in intensive care unit].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013 Mar;25(3):167-70. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013. PMID: 23798067 Clinical Trial. Chinese.
-
Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497].Crit Care. 2005 Jun;9(3):R200-10. doi: 10.1186/cc3495. Epub 2005 Mar 15. Crit Care. 2005. PMID: 15987391 Free PMC article. Clinical Trial.
-
[The use of remifentanil in critically ill patients. Clinical findings and early experience].Anaesthesist. 1999 Sep;48(9):625-9. doi: 10.1007/s001010050762. Anaesthesist. 1999. PMID: 10525595 Clinical Trial. German.
-
Analgosedation: a paradigm shift in intensive care unit sedation practice.Ann Pharmacother. 2012 Apr;46(4):530-40. doi: 10.1345/aph.1Q525. Epub 2012 Apr 10. Ann Pharmacother. 2012. PMID: 22496477 Review.
-
Effectiveness of dexmedetomidine versus propofol on extubation times, length of stay and mortality rates in adult cardiac surgery patients: a systematic review and meta-analysis.JBI Database System Rev Implement Rep. 2018 May;16(5):1220-1239. doi: 10.11124/JBISRIR-2017-003488. JBI Database System Rev Implement Rep. 2018. PMID: 29762314
References
-
- Optimal sedation in pediatric intensive care patients: a systematic review. Vet NJ, Ista E, de Wildt SN, van Dijk M, Tibboel D, de Hoog M. Intensive Care Med. 2013;39:1524–1534. - PubMed
-
- Predictors of mortality in patients with suspected propofol infusion syndrome. Fong JJ, Sylvia L, Ruthazer R, Schumaker G, Kcomt M, Devlin JW. Crit Care Med. 2008;36:2281–2287. - PubMed
-
- Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Hughes MA, Glass PS, Jacobs JR. Anesthesiology. 1992;76:334–341. - PubMed
-
- Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: a first evaluation. Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M. Crit Care Med. 2008;36:2427–2432. - PubMed
-
- Remifentanil/midazolam versus fentanyl/midazolam for analgesia and sedation of mechanically ventilated neonates and young infants: a randomized controlled trial. Welzing L, Oberthuer A, Junghaenel S, Harnischmacher U, Stützer H, Roth B. Intensive Care Med. 2012;38:1017–1024. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
