Update on the pathogenesis of endometriosis-related infertility based on contemporary evidence
- PMID: 40130159
- PMCID: PMC11930837
- DOI: 10.3389/fendo.2025.1558271
Update on the pathogenesis of endometriosis-related infertility based on contemporary evidence
Abstract
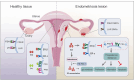
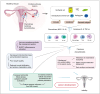
Endometriosis, the most prevalent cause of infertility, is associated with anatomical distortion leading to adhesions and fibrosis, as well as endocrine abnormalities and immune disorders. This review discusses the mechanisms underlying endometriosis-related infertility. Firstly, alterations in the hypothalamic-pituitary-ovarian axis lead to the secretion of gonadotropins and steroid hormones, with adverse effects on ovulation and implantation, leading to fertility decline. Secondly, dysregulation of the hypothalamic-pituitary-adrenal axis induces elevated serum cortisol and prolactin levels in patients with endometriosis, accounting for its regulation of stress, depression, and anxiety. Abnormal interactions between endometrial cells and the immune system change the local microenvironment, resulting in epithelial-mesenchymal transition and inflammation. Activated epithelial cells, stromal cells, and immunocytes produce various chemokines, cytokines, or autoantibodies, creating an unfavorable environment for embryo implantation. These findings suggest that alterations in the immune spectrum play a crucial role in endometriosis-related infertility. Thirdly, oxidative stress has adverse effects on the ovarian reserve and subsequent embryonic development, predicting another promising strategy for endometriosis-related infertility. An unbalanced redox state, including impaired mitochondrial function, dysregulated lipid metabolism, and iron-induced oxidative stress, generates a pro-oxidative microenvironment, which negatively impacts oocyte quality and sperm and embryo viability. Thus, an updated understanding of the mechanisms involved in this disease will help to develop effective strategies to manage endometriosis-related infertility.
Keywords: endocrine; endometriosis; immune dysfunction; infertility; oxidative stress.
Copyright © 2025 Qi, Li, Chen, Luo, Zhou, Zhou, Zhang, Chen and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Ethiopathogenic mechanisms of endometriosis-related infertility.JBRA Assist Reprod. 2019 Aug 22;23(3):273-280. doi: 10.5935/1518-0557.20190029. JBRA Assist Reprod. 2019. PMID: 31091056 Free PMC article. Review.
-
Novel therapeutic targets to improve IVF outcomes in endometriosis patients: a review and future prospects.Hum Reprod Update. 2021 Aug 20;27(5):923-972. doi: 10.1093/humupd/dmab014. Hum Reprod Update. 2021. PMID: 33930149 Review.
-
Upregulated let-7 expression in the follicular fluid of patients with endometriomas leads to dysfunction of granulosa cells through targeting of IGF1R.Hum Reprod. 2025 Jan 1;40(1):119-137. doi: 10.1093/humrep/deae247. Hum Reprod. 2025. PMID: 39521729 Free PMC article.
-
Cellular and molecular basis for endometriosis-associated infertility.Cell Tissue Res. 2012 Sep;349(3):849-62. doi: 10.1007/s00441-011-1309-0. Cell Tissue Res. 2012. PMID: 22298022 Free PMC article. Review.
-
Oxidative stress and oocyte quality: ethiopathogenic mechanisms of minimal/mild endometriosis-related infertility.Cell Tissue Res. 2016 Apr;364(1):1-7. doi: 10.1007/s00441-015-2339-9. Epub 2015 Dec 19. Cell Tissue Res. 2016. PMID: 26685866 Review.
Cited by
-
Endometriotic Follicular Fluid Affects Granulosa Cells' Morphology and Increases Duplication Rate and Connexin-43 Expression.Biomolecules. 2025 Apr 10;15(4):561. doi: 10.3390/biom15040561. Biomolecules. 2025. PMID: 40305294 Free PMC article.
-
Beyond pelvic pathology: retinal microvascular rarefaction as a systemic marker in endometriosis.BMC Womens Health. 2025 Jul 10;25(1):343. doi: 10.1186/s12905-025-03899-6. BMC Womens Health. 2025. PMID: 40640800 Free PMC article.
-
Leveraging epigenetic aberrations in the pathogenesis of endometriosis: from DNA methylation to non-coding RNAs.Front Genet. 2025 Jul 28;16:1597287. doi: 10.3389/fgene.2025.1597287. eCollection 2025. Front Genet. 2025. PMID: 40792071 Free PMC article. Review.
References
-
- Anupa G, Sharma JB, Roy KK, Sengupta J, Ghosh D. An assessment of the multifactorial profile of steroid-metabolizing enzymes and steroid receptors in the eutopic endometrium during moderate to severe ovarian endometriosis. Reprod Biol Endocrinol. (2019) 17:111. doi: 10.1186/s12958-019-0553-0 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

