Recent advances in allylation of chiral secondary alkylcopper species
- PMID: 40130179
- PMCID: PMC11931642
- DOI: 10.3762/bjoc.21.51
Recent advances in allylation of chiral secondary alkylcopper species
Abstract
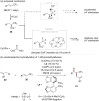
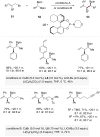
The transition-metal-catalyzed asymmetric allylic substitution represents a pivotal methodology in organic synthesis, providing remarkable versatility for complex molecule construction. Particularly, the generation and utilization of chiral secondary alkylcopper species have received considerable attention due to their unique properties in stereoselective allylic substitution. This review highlights recent advances in copper-catalyzed asymmetric allylic substitution reactions with chiral secondary alkylcopper species, encompassing several key strategies for their generation: stereospecific transmetalation of organolithium and organoboron compounds, copper hydride catalysis, and enantiotopic-group-selective transformations of 1,1-diborylalkanes. Detailed mechanistic insights into stereochemical control and current challenges in this field are also discussed.
Keywords: allylic substitution; chiral secondary organocopper; copper-mediated reaction; stereoselectivity.
Copyright © 2025, Kim et al.
Figures

















References
-
- Hartwig J F, Pouy M J. Top Organomet Chem. 2011;34:169–208. doi: 10.1007/978-3-642-15334-1_7. - DOI
Publication types
LinkOut - more resources
Full Text Sources
