Voluntary locomotion induces an early and remote hemodynamic decrease in the large cerebral veins
- PMID: 40130194
- PMCID: PMC11931294
- DOI: 10.1117/1.NPh.12.S1.S14609
Voluntary locomotion induces an early and remote hemodynamic decrease in the large cerebral veins
Abstract
Significance: Behavior regulates dural and cerebral vessels, with spontaneous locomotion inducing dural vessel constriction and increasing stimulus-evoked cerebral hemodynamic responses. It is vital to investigate the function of different vascular network components, surrounding and within the brain, to better understand the role of the neurovascular unit in health and neurodegeneration.
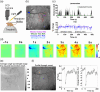
Aim: We characterized locomotion-induced hemodynamic responses across vascular compartments of the whisker barrel cortex: artery, vein, parenchyma, draining, and meningeal vein.
Approach: Using 2D-OIS, hemodynamic responses during locomotion were recorded in 9- to 12-month-old awake mice: wild-type, Alzheimer's disease (AD), atherosclerosis, or mixed (atherosclerosis/AD) models. Within the somatosensory cortex, responses were taken from pial vessels inside the whisker barrel region [(WBR): "whisker artery" and "whisker vein"], a large vein from the sagittal sinus adjacent to the WBR (draining vein), and meningeal vessels from the dura mater (which do not penetrate cortical tissue).
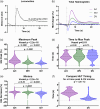
Results: We demonstrate that locomotion evokes an initial decrease in total hemoglobin (HbT) within the draining vein before the increase in HbT within WBR vessels. The locomotion event size influences the magnitude of the HbT increase in the pial vessels of the WBR but not of the early HbT decrease within the draining veins. Following locomotion onset, an early HbT decrease was also observed in the overlying meningeal vessels, which unlike within the cortex did not go on to exceed baseline HbT levels during the remainder of the locomotion response. We show that locomotion-induced hemodynamic responses are altered in disease in the draining vein and whisker artery, suggesting this could be an important neurodegeneration biomarker.
Conclusions: This initial reduction in HbT within the draining and meningeal veins potentially serves as a "space-saving" mechanism, allowing for large increases in cortical HbT associated with locomotion. Given this mechanism is impacted by disease, it may provide an important target for vascular-based therapeutic interventions.
Keywords: Alzheimer’s disease; hemodynamic; neurodegeneration; optical imaging spectroscopy; vasculature.
© 2025 The Authors.
Figures



References
LinkOut - more resources
Full Text Sources

