Cutaneous Evaluation of Fe3O4 Nanoparticles: An Assessment Based on 2D and 3D Human Epidermis Models Under Standard and UV Conditions
- PMID: 40130196
- PMCID: PMC11932040
- DOI: 10.2147/IJN.S513423
Cutaneous Evaluation of Fe3O4 Nanoparticles: An Assessment Based on 2D and 3D Human Epidermis Models Under Standard and UV Conditions
Abstract
Purpose: The high-speed development of nanotechnology industry has fueled a plethora of engineered nanoparticles (NPs) and NP-based consumer products, further leading to massive and uncontrolled human exposure. In this regard, the researches addressing the safety assessment of NPs should be re-approached from the perspective of test parameters variety, closely simulating daily life scenarios. Therefore, the present study adopts complex in vitro models to establish the safety profile of Fe3O4 NPs, by using 2D and 3D human epidermis models under both standard and UV exposure conditions.
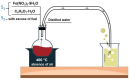
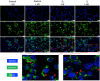
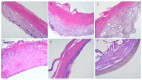
Methods: Advanced 3D human reconstructed epidermal tissues and two different monolayers of immortalized human cells (keratinocytes and fibroblasts), using series of in vitro assays were employed in the current study to evaluate multiple biological responses, as follows: i) divers protocols (skin irritation, phototoxicity assay); ii) different conditions (± UV exposure) and iii) a wide variety of quantification methods, such as: MTT, NR and LDH colorimetric tests - performed to evaluate the viability of the cells/microtissues, respectively, the cytotoxicity of the test compounds. In addition, IL-1α ELISA assay was used to quantify the inflammatory activity induced by the test samples, while immunocytochemistry analysis through fluorescent microscopy was employed to provide insightful information regarding the possible mechanism of action of test samples.
Results: The two test samples (S1 and S2) induced a higher cell viability decrease on immortalized human keratinocytes (HaCaT) compared to human fibroblasts (1BR3), while 3D-epidermis microtissues showed similar viabilities when treated with both samples under standard conditions (-UV rays) - for both type of evaluation protocols: skin irritation and phototoxicity. However, UV irradiation of 3D-microtissues pre-exposed to test samples led to different results between the two test samples, revealing that S2 sample induced a significant impairment of human epidermis viability, whereas S1 sample elicited an activity similar to the one recorded under standard conditions (-UV).
Conclusion: The present results indicate significant differences in toxicity between the two in vitro models under UV conditions, highlighting the importance of model selection and exposure parameters in assessing NP safety. Thus, our findings suggest that Fe3O4 NPs may pose some risks under specific environmental conditions, within the limitations of the experimental setup, and further research is needed to refine safety guidelines.
Keywords: 1BR3; 3D-microtissue; HaCaT; UV; cytotoxicity; magnetite NPs.
© 2025 Watz et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- McCracken C, Dutta PK, Waldman WJ. Critical assessment of toxicological effects of ingested nanoparticles. Environ Sci Nano. 2016;3(2):256–282.
-
- Askri D, Ouni S, Galai S, et al. Nanoparticles in foods? A multiscale physiopathological investigation of iron oxide nanoparticle effects on rats after an acute oral exposure: trace element biodistribution and cognitive capacities. Food and Chemical Toxicology. 2019;127:173–181. doi:10.1016/j.fct.2019.03.006 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

