XBP1s-EDEM2 Prevents the Onset and Development of HFpEF by Ameliorating Cardiac Lipotoxicity
- PMID: 40130322
- PMCID: PMC12124211
- DOI: 10.1161/CIRCULATIONAHA.124.072194
XBP1s-EDEM2 Prevents the Onset and Development of HFpEF by Ameliorating Cardiac Lipotoxicity
Abstract
Background: Morbidity and mortality of heart failure with preserved ejection fraction (HFpEF) is increased in metabolic disorders. However, options for preventing and treating these prevalent outcomes are limited. Intramyocardial lipotoxicity contributes to cardiac dysfunction. Here, we investigate the mechanisms underlying EDEM2 (endoplasmic reticulum degradation-enhancing alpha-mannosidase-like protein 2) regulation of cardiac lipid homeostasis and assess strategies that inhibit the incidence and progression of HFpEF.
Methods: Metabolic stress was induced in C57BL/6 male mice using a high-fat diet and Nω-nitro-L-arginine methyl ester. The recombinant adeno-associated virus 9 delivery system was used for loss- and gain-of-function studies. Palmitic acid and oleic acid stimulation of rat cardiomyocytes and human induced pluripotent stem cell-derived cardiomyocytes imitated a condition of high lipids in vitro. Molecular mechanisms were investigated via RNA sequencing, mass spectrometry proteomics, lipidomic analyses, transmission electron microscopy, histology, and luciferase reporter assays.
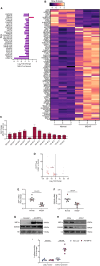
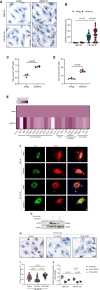
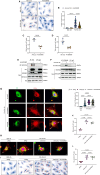
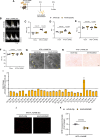
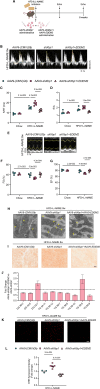
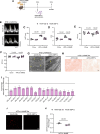
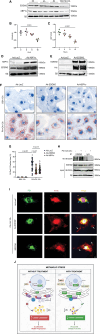
Results: In the human heart, we first detected lipid overload accompanied by a reduction of XBP1 (X-box binding protein 1) under metabolic stress. Thereafter, a decrease in EDEM2 was confirmed in human and mouse HFpEF hearts. Given that XBP1s (spliced X-box binding protein 1) is a transcription factor, EDEM2 was identified as its new target in cardiomyocytes. EDEM2 knockdown mice manifested lipid droplet accumulation and higher levels of triglycerides and diglycerides in the myocardium, aggravating oxidative stress, hypertrophy, and the onset and progression of HFpEF under metabolic stress. XBP1s ablation mice displayed a similar myocardial lipid disturbance and cardiac phenotypes, which were reversed by EDEM2 overexpression. Mechanistically, the findings obtained from rat cardiomyocytes and human induced pluripotent stem cell-derived cardiomyocytes demonstrated that, in the presence of EDEM2, SEC23A mediated intracellular translocation of ATGL (adipose triglyceride lipase) under fatty acid stimulation, inhibiting ATGL degradation and excessive intracellular lipid droplets. Furthermore, the functional studies supported that EDEM2 prevention of lipid overload occurred in an ATGL-dependent manner. Therapeutically, cardiac XBP1s or EDEM2 restoration mitigated lipid deposition and preserved lipid profiles in the myocardium, thus preventing the development of HFpEF.
Conclusions: We demonstrate a cardioprotective mechanism regulating myocardial lipid homeostasis. The findings provide a promising therapeutic target to prevent and treat HFpEF, a condition with limited treatment options.
Keywords: EDEM2; HFpEF; XBP1s; cardiac lipotoxicity; heart failure; metabolic stress.
Conflict of interest statement
T.M.A.M. holds equities at Tenaya Therapeutics.
Figures

References
-
- Karwi QG, Ho KL, Pherwani S, Ketema EB, Sun Q, Lopaschuk GD. Concurrent diabetes and heart failure: interplay and novel therapeutic approaches. Cardiovasc Res. 2022;118:686–715. doi: 10.1093/cvr/cvab120 - PubMed
-
- Schiattarella GG, Altamirano F, Kim SY, Tong D, Ferdous A, Piristine H, Dasgupta S, Wang X, French KM, Villalobos E, et al. Xbp1s-FoxO1 axis governs lipid accumulation and contractile performance in heart failure with preserved ejection fraction. Nat Commun. 2021;12:1684. doi: 10.1038/s41467-021-21931-9 - PMC - PubMed
-
- Zlobine I, Gopal K, Ussher JR. Lipotoxicity in obesity and diabetes-related cardiac dysfunction. Biochim Biophys Acta. 2016;1861:1555–1568. doi: 10.1016/j.bbalip.2016.02.011 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

