Music exposure enhances resistance to Salmonella infection by promoting healthy gut microbiota
- PMID: 40130867
- PMCID: PMC12054044
- DOI: 10.1128/spectrum.02377-24
Music exposure enhances resistance to Salmonella infection by promoting healthy gut microbiota
Abstract
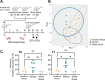
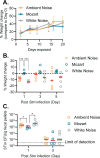
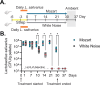
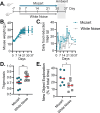
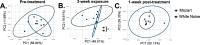
Music intervention is gaining recognition as a cost-effective therapeutic for improving human health. Despite its growing application, the mechanisms through which music exerts beneficial health effects remain largely unexplored. Here, we show that music can exert beneficial effects in mice through modulating gut microbiome composition. Adult mice were exposed to ambient noise, Mozart's Flute Quartet in D Major, K. 285, or white noise over a three-week period. Afterward, we observed treatment-specific changes in the community of gut commensal bacteria in these animals. Upon subsequent challenge with the bacterial pathogen Salmonella typhimurium, control groups exhibited significant weight loss and increased Salmonella colonization, whereas the Mozart-treated group did not. 16S ribosomal RNA gene sequencing revealed that the Mozart group showed a significant increase in Lactobacillus salivarius, a probiotic known for its antibacterial properties. Further experiments confirmed that L. salivarius mitigated Salmonella infection in mice and that L. salivarius acidified local environments in in vitro culture, thus inhibiting Salmonella growth. Additionally, mice exposed to Mozart consumed more food but showed similar body weight compared to the control groups. Behavioral assessments, including open field and object location tests, revealed that Mozart-treated mice were more active, less anxious, and exhibited enhanced spatial memory. Finally, Mozart exposure was shown to significantly boost colonization of administered L. salivarius and alter gut metabolite profiles. These findings suggest that music exposure fosters healthier gut microbiota, enhancing resistance to bacterial infections and highlighting the potential of music therapy as a novel strategy to combat drug-resistant pathogen infections.
Importance: Music therapy is increasingly recognized as a low-cost approach to improving health, but how it works remains unclear. Our study demonstrates that music can positively influence health by altering the gut microbiome. In a mouse model, exposure to Mozart's Flute Quartet in D Major enhanced the gut microbiota, specifically increasing levels of the beneficial bacterium Lactobacillus salivarius. This probiotic protected mice from Salmonella infection by creating an acidic environment that inhibited pathogen growth. Mozart-treated mice also showed reduced anxiety, better spatial memory, and higher food intake without weight gain, suggesting the benefits of music exposure. These findings reveal a novel link between music, gut health, and disease resistance, suggesting that music therapy could be a promising strategy for enhancing gut microbiota and combating infections, including those caused by drug-resistant bacteria.
Keywords: Lactobacillus; Salmonella; metabolomics; microbiota; music therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Probiotic alleviate fluoride-induced memory impairment by reconstructing gut microbiota in mice.Ecotoxicol Environ Saf. 2021 Jun 1;215:112108. doi: 10.1016/j.ecoenv.2021.112108. Epub 2021 Mar 30. Ecotoxicol Environ Saf. 2021. PMID: 33799132
-
Gut Microbial and Metabolic Responses to Salmonella enterica Serovar Typhimurium and Candida albicans.mBio. 2018 Nov 6;9(6):e02032-18. doi: 10.1128/mBio.02032-18. mBio. 2018. PMID: 30401779 Free PMC article.
-
Ligilactobacillus salivarius XP132 with antibacterial and immunomodulatory activities inhibits horizontal and vertical transmission of Salmonella Pullorum in chickens.Poult Sci. 2024 Oct;103(10):104086. doi: 10.1016/j.psj.2024.104086. Epub 2024 Jul 10. Poult Sci. 2024. PMID: 39098298 Free PMC article.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection.Therap Adv Gastroenterol. 2022 Nov 18;15:17562848221134396. doi: 10.1177/17562848221134396. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 36425405 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

