Cellular SLC35B4 promotes internalization during influenza A virus entry
- PMID: 40130891
- PMCID: PMC12077083
- DOI: 10.1128/mbio.00194-25
Cellular SLC35B4 promotes internalization during influenza A virus entry
Abstract
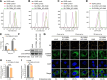
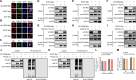
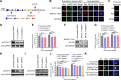
SLC35B4, a nucleotide sugar transporter that mediates the transport of UDP-GlcNAc and UDP-xylose, was found to be required for the replication of influenza A virus (IAV) of the H5N1 subtype in our genome-wide siRNA library screen. We found that defective IAV replication in SLC35B4-deficient A549 cells was independent of virus strain specificity, and the virulence of IAV in Slc35b4 knockdown mice was also decreased. By examining the individual stages of the IAV replication cycle, we discovered that the amount of internalized IAV was significantly reduced in SLC35B4-knockout A549 cells. Mechanistically, SLC35B4 facilitated IAV replication by transporting UDP-xylose, which attaches to the serine residue of heparan sulfate proteoglycans (HSPGs) in the heparan sulfate (HS) biosynthesis pathway. Knockdown of associated host factors (i.e., XYLT2, B4GALT7, EXT1, and EXT2) in the HS biosynthesis pathway also impaired IAV replication. Furthermore, we revealed that AGRN, a unique HSPG family member, was important for the endocytosis of IAV in A549 cells. Moreover, we found that the homeostasis of the AGRN protein was regulated by HS modification mediated by the initial UDP-xylose transporter SLC35B4, thereby affecting the expression level of endocytic adapter AP2B1 to influence IAV internalization. Collectively, these findings establish that SLC35B4 is an important regulator of IAV replication and uncover the underlying mechanisms by which SLC35B4 employs UDP-xylose transport activity to promote IAV internalization.IMPORTANCEThe entry process of IAV represents a favorable target for drug development. In this study, we identified SLC35B4 as an important host factor for the efficient replication of different subtypes of IAV in vitro and for the virulence of IAV in mice. We revealed that SLC35B4 employed its UDP-xylose transport activity to promote the HS biosynthesis pathway, thereby assisting IAV internalization into target cells in the early stage of viral infection. Consistently, several downstream factors in the HS biosynthesis pathway, i.e., XYLT2, B4GALT7, EXT1, and EXT2, as well as a specific HSPG member AGRN were also important for the replication of IAV. Furthermore, the UDP-xylose-transporting activity of SLC35B4 was involved in the regulation of the homeostasis of the AGRN protein by HS modification, which influenced virus internalization by affecting the expression levels of AP2B1. Together, the identification of the SLC35B4-XYLT2-B4GALT7-EXT1-EXT2-AGRN-AP2B1 axis may shed light on the development of potential anti-IAV therapeutics.
Keywords: AGRN; AP2B1; SLC35B4; heparan sulfate modification; influenza A virus; internalization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
The G Protein-Coupled Receptor FFAR2 Promotes Internalization during Influenza A Virus Entry.J Virol. 2020 Jan 6;94(2):e01707-19. doi: 10.1128/JVI.01707-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31694949 Free PMC article.
-
Prolidase is required for early trafficking events during influenza A virus entry.J Virol. 2014 Oct;88(19):11271-83. doi: 10.1128/JVI.00800-14. Epub 2014 Jul 16. J Virol. 2014. PMID: 25031340 Free PMC article.
-
Genome-wide CRISPR/Cas9 Screen Identifies Host Factors Essential for Influenza Virus Replication.Cell Rep. 2018 Apr 10;23(2):596-607. doi: 10.1016/j.celrep.2018.03.045. Cell Rep. 2018. PMID: 29642015 Free PMC article.
-
Entry of influenza A virus into host cells - recent progress and remaining challenges.Curr Opin Virol. 2021 Jun;48:23-29. doi: 10.1016/j.coviro.2021.03.001. Epub 2021 Apr 7. Curr Opin Virol. 2021. PMID: 33838498 Review.
-
The role of host cell Rab GTPases in influenza A virus infections.Future Microbiol. 2021 Apr;16:445-452. doi: 10.2217/fmb-2020-0092. Epub 2021 Apr 13. Future Microbiol. 2021. PMID: 33847136 Review.
Cited by
-
CircMYO9A inhibits influenza A virus replication by dampening haemagglutinin cleavage via increasing SERPINE1/PAI-1 expression.Emerg Microbes Infect. 2025 Dec;14(1):2502007. doi: 10.1080/22221751.2025.2502007. Epub 2025 May 20. Emerg Microbes Infect. 2025. PMID: 40314425 Free PMC article.
References
-
- Eisfeld AJ, Biswas A, Guan L, Gu C, Maemura T, Trifkovic S, Wang T, Babujee L, Dahn R, Halfmann PJ, Barnhardt T, Neumann G, Suzuki Y, Thompson A, Swinford AK, Dimitrov KM, Poulsen K, Kawaoka Y. 2024. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature 633:426–432. doi:10.1038/s41586-024-07766-6 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2021YFD1800203/National Key Research and Development Program of China
- 2021YFD1800204/National Key Research and Development Program of China
- 32272979/National Natural Science Foundation of China
- 32192453/National Natural Science Foundation of China
- 32172847/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
