Endothelial JCAD Worsens Acute Ischemic Stroke Outcomes by Enhancing Inflammation in Response to Ischemia/Reperfusion
- PMID: 40131119
- PMCID: PMC11897444
- DOI: 10.1016/j.jacbts.2024.09.009
Endothelial JCAD Worsens Acute Ischemic Stroke Outcomes by Enhancing Inflammation in Response to Ischemia/Reperfusion
Abstract
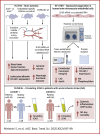
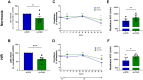
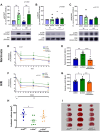
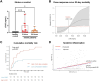
The role of junctional protein associated with coronary artery disease (JCAD) in acute ischemic stroke (AIS) has not been investigated yet. To investigate its potential as a therapeutic target, transient middle cerebral artery occlusion was induced in JCAD knockout mice, with improvement of stroke outcome and reduced blood-brain barrier permeability and expression of vascular cell adhesion molecule (VCAM)-1. JCAD plays a deleterious role in ischemia/reperfusion cerebral damage and associates with higher 90-day mortality in patients with AIS. JCAD may thus represent a novel prognostic biomarker for patients with AIS, as well as a therapeutic target.
Keywords: acute ischemic stroke; blood-brain barrier; endothelial cells; hypoxia/reoxygenation; inflammation.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was funded by Swiss Heart Foundation grant FF22014/2022 (Dr Ministrini), the Novartis Foundation for Medical-Biological Research grant 21B070 (Prof Liberale), and Swiss National Science Foundation grant 501100001711-197510] (Prof Camici). Dr Puspitasari is the recipient of a Forschungskredit Candoc grant from the University of Zurich and a grant from Swiss Life Foundation for Public Health and Medical Research. Dr Kraler has received institutional research grants from the Jubiläumsstiftung SwissLife, the Lindenhof Foundation, the Novartis Foundation for Medical-Biological Research, the Swiss Heart Foundation, the Swiss Society of Cardiology, and the Theodor-Ida-Herzog-Egli Foundation; has received equipment and materials from Roche Diagnostics outside the submitted work; and has received travel support from the European Atherosclerosis Society, the European Society of Cardiology, the European Society of Clinical Investigation, Sphingotec GmbH, the 4TEEN4 Pharmaceuticals GmbH, and PAM Theragnostics GmbH. Dr Wenzl has received financial support from the Foundation for Cardiovascular Research-Zurich Heart House, the Lindenhof Foundation, the European Society of Cardiology, the Swiss Heart Foundation, the Fonds zur Förderung des akademischen Nachwuchses of the University of Zurich, the Medical University of Graz, the Theodor-Ida-Herzog-Egli Foundation, the Sphingotec GmbH, the 4TEEN4 Pharmaceuticals GmbH, and the PAM Theragnostics GmbH outside this work. Prof Katan has received funding from the Swiss National Science Foundation (Project Nr. 182267 and Project Nr. 213471), the Swiss Heart Foundation, and in kind contributions from ROCHE Diagnostics and BRHAMS Thermofisher, outside the submitted work. Prof Montecucco is the recipient of Rete CARDIOLOGICA grant RCR-2022-23682288. Work supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) - (DN. 1553 11.10.2022) to Prof. Montecucco Prof Lüscher holds leadership positions at the European Society of Cardiology, Swiss Heart Foundation, and the Foundation for Cardiovascular Research-Zurich Heart House; has received institutional educational and research grants outside this work from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daichi Sankyo, Eli Lilly, Novartis. Novo Nordisk, Sanofi, Servier, and Vifor; and has received consulting fees from Dacadoo, Novartis, Novo Nordisk, Pfizer, and Philips. Prof Camici and Prof Liberale are coinventors on the International Patent WO/2020/226993 filed in April 2020; the patent relates to the use of antibodies which specifically bind interleukin-1a to reduce various sequelae of ischemia-reperfusion injury to the central nervous system. Prof Camici is a consultant to Sovida Solutions limited; is the recipient of a Sheikh Khalifa’s Foundation Assistant Professorship at the Faculty of Medicine, University of Zurich; and has received financial support by the Alfred and Annemarie von Sick Grants for Translational and Clinical Research Cardiology and Oncology and by the Swiss Heart Foundation. Prof Liberale has received financial support from the Swiss Heart Foundation and the Novartis Foundation for Medical-Biological Research. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Powers W.J., Rabinstein A.A., Ackerson T., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. - PubMed
-
- Emberson J., Lees K.R., Lyden P., et al. Stroke Thrombolysis Trialists’ Collaborative Group Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. - PMC - PubMed
-
- Q8P266 – JCAD_HUMAN. UniProt. Accessed December 5, 2023 https://www.uniprot.org/uniprotkb/Q9P266/entry
LinkOut - more resources
Full Text Sources
Miscellaneous

