Impact of MEK Inhibition on Childhood RASopathy-Associated Hypertrophic Cardiomyopathy
- PMID: 40131150
- PMCID: PMC11897442
- DOI: 10.1016/j.jacbts.2024.10.002
Impact of MEK Inhibition on Childhood RASopathy-Associated Hypertrophic Cardiomyopathy
Abstract
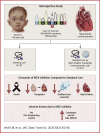
There is an unmet medical need to treat patients with severe hypertrophic cardiomyopathy leading to heart failure and death in children carrying pathogenic activating variants in the RAS/mitogen-activated protein kinase pathway. A retrospective analysis of 61 patients provides evidence for decreased mortality and morbidity with improved cardiac status in patients with RASopathy with severe hypertrophic cardiomyopathy receiving mitogen-activated protein kinase kinase inhibition (n = 30) vs those with standard-of-care treatment (n = 31). Side effects were not life threatening and were manageable. The data presented suggest that personalized therapies targeting underlying signaling pathway abnormalities might be effective in critically ill patients with RASopathy warranting clinical investigation.
Keywords: MEK inhibition; Noonan syndrome spectrum; RAS/MAPK signaling; RASopathies; childhood cardiomyopathy; survival.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Dr Wolf is supported by the Gerd-Killian Award of Deutsche Herzstiftung, Deutsches Zentrum für Herz-Kreislauf-Forschung (German Center for Cardiovascular Research) and by Bundesministerium für Bildung und Forschung (German Ministry of Education and Research); and is member of the European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart. Dr Zenker has received funding from Bundesministerium für Bildung und Forschung as part of national and European research networks on RASopathies (German Network for RASopathy Research; FKZ: 01GM1902A) and European Joint Programme on Rare Disease funding (NSEuroNet consortium; FKZ: 01GM1921A). Several authors of this publication are members of the European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability, which is funded by the EU4Health program of the European Union, under grant agreement 101085231 (National Children’s Research Centre). Dr Norrish is supported by the Association for European Paediatric and Congenital Cardiology and Great Ormond Street Hospital Charity. Dr Kaski is supported by a Medical Research Council Clinical Academic Partnership Award and by the British Heart Foundation, Action Medical Research, Max's Foundation, and the Great Ormond Street Hospital Children's Charity. Dr Gelb is supported by a grant from the National Institutes of Health (HL135742). Dr Andelfinger is supported by the Banque Nationale Research Excellence Chair in Cardiovascular Genetics. None of the sponsors or funders had any role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; and in the preparation, review, or approval of the manuscript. Dr Wolf has received honoraria from Novo Nordisk and Bristol Myers Squibb; is a consultant for Bristol Myers Squibb, Rocket Pharmaceuticals, Day One Biopharmaceuticals, BioMarin Pharmaceuticals, Adrenomed, and Pliant Therapeutics. Dr Zenker has received honoraria: Novo Nordisk; and is a consultant or advisory board member for Day One Biopharmaceuticals, Novo Nordisk, and Novartis. Dr Friede has received personal fees from Actimed, Aslan, Bayer, Biosense Webster, Bristol Myers Squibb, CSL Behring, Enanta, Fresenius Kabi, Galapagos, Immunic, IQVIA, Janssen, Johnson & Johnson Medical, KyowaKirin, LivaNova, Minoryx, Novartis, RECARDIO, Recordati, Relaxera, Roche, Servier, Viatris, VICO Therapeutics, and Vifor for statistical consultancies, including data monitoring committees, all outside the submitted work. Dr Kaski has received honoraria from Novo Nordisk; and is a consultant for Novo Nordisk. Dr Gelb is a named inventor on issued patents related to PTPN11, SHOC2, RAF1, and SOS1 mutations. The Icahn School of Medicine at Mount Sinai licensed the patents to several diagnostics companies (Correlegan, GeneDx, LabCorp, and Prevention Genetics) and has received royalty payments, some of which are distributed to Dr Gelb. Dr Gelb was the recipient of a sponsored research agreement from Onconova Therapeutics; and was a consultant for Day One Biopharmaceuticals and BioMarin Pharmaceuticals. Dr Andelfinger is a consultant for Day One Biopharmaceuticals and BioMarin Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Wilkinson J.D., Lowe A.M., Salbert B.A., et al. Outcomes in children with Noonan syndrome and hypertrophic cardiomyopathy: a study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2012;164:442–448. - PubMed
-
- Roberts AE. Noonan syndrome. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, eds. GeneReviews. Seattle: University of Washington. - PubMed
-
- Prendiville T.W., Gauvreau K., Tworog-Dube E., et al. Cardiovascular disease in Noonan syndrome. Arch Dis Childhood. 2014;99:629–634. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

