Therapeutic Efficacy of a Novel Pharmacologic GRK2 Inhibitor in Multiple Animal Models of Heart Failure
- PMID: 40131155
- PMCID: PMC11897459
- DOI: 10.1016/j.jacbts.2024.10.008
Therapeutic Efficacy of a Novel Pharmacologic GRK2 Inhibitor in Multiple Animal Models of Heart Failure
Abstract
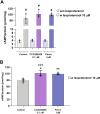
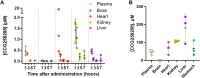
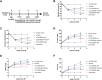
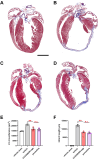
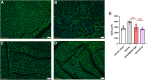
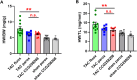
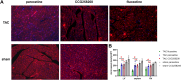
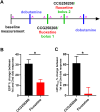
GRK2 is the most prominent G protein-coupled receptor kinase that is upregulated in heart failure (HF), and inhibiting GRK2 has improved cardiac function in mice. CCG258208, generated from the paroxetine scaffold, which has GRK2 inhibitory properties, has a 50-fold higher selectivity for GRK2 at 100-fold lower doses. We evaluated CCG258208 in 2 mice HF models and found that CCG258208 has robust therapeutic effects. In a chronic mini-swine HF model, acute administration of CCG258208 enhanced dobutamine inotropic responses. Our results indicate that CCG258208 has robust cardioprotective and HF-reversing effects in different HF models and it stands as a promising lead for HF therapy.
Keywords: GPCR; GRK2; LV function; cardiac pathophysiology; dobutamine; heart failure; paroxetine.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was funded by National Institutes of Health grants R01 HL061690 and R01 HL157151 (to Dr Koch), AHA Merit Award (to Dr Koch) R01 HL071818 (to Drs Koch and Tesmer), and National Institutes of Health RO1 grants CA254402 and CA221289 (to Dr Tesmer). Funding for pharmacokinetics was provided by the PIDD (Purdue) FY20 Drug Evaluation Committee Project “Selective Inhibition of G Protein-Coupled Receptor Kinases for the Treatment of Heart Failure.” The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





References
-
- Virani S.S., Alonso A., Benjamin E.J., et al. Heart Disease and Stroke Statistics-2020 Update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. - PubMed
-
- Cohn J.N., Archibald D.G., Ziesche S., et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–1552. - PubMed
-
- Massie B.M. 15 years of heart-failure trials: what have we learned? Lancet. 1998;352(Suppl 1):SI29–SI33. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

