Phenotypic and functional dysregulations of CD8 + T Cells in myasthenia gravis
- PMID: 40131529
- PMCID: PMC11937161
- DOI: 10.1007/s10238-025-01603-4
Phenotypic and functional dysregulations of CD8 + T Cells in myasthenia gravis
Abstract
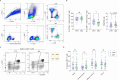
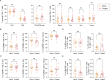
Myasthenia Gravis (MG) is a heterogeneous autoimmune disorder characterized by fluctuating muscle weakness caused by autoantibodies targeting neuromuscular junction components. While the role of CD4 + T cells in MG is well established, the contribution of CD8 + T cells remains poorly understood. In this study, we analyze CD8 + T cells in 36 MG patients and 38 age- and gender-matched controls using flow cytometry to evaluate subset distribution, granzyme expression, and cytokine production. MG patients exhibit an altered CD4 + /CD8 + T cell ratio and significant changes in CD8 + T cell subsets, including increased central memory CD8 + T cell (Tcm) proportions and decreased effector memory CD8 + T cell (Tem) proportions. Granzyme B expression in Tcm cells is significantly elevated in MG patients, whereas no significant changes are observed in other subsets or GZMK expression. Cytokine analysis reveals increased IL-21, GM-CSF, and IL-17A production by CD8 + T cells in MG patients. These phenotypic and functional alterations of CD8 + T cells persist during the acute phase of the disease, regardless of immunotherapy usage, and vary between ocular and generalized MG. Subgroup and correlation analyses further identify age-dependent and age-independent dysregulations of CD8 + T cells, indicating complex and subtype-specific roles of CD8 + T cells in the immunopathological processes underlying MG. Our findings provide novel insights into the involvement of CD8 + T cells in MG pathogenesis, laying a foundation for future research and potential therapeutic strategies targeting CD8 + T cells.
Keywords: CD8 + T cells; GM-CSF; Granzyme B; IL-21; Myasthenia gravis; Pathogenesis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments were performed in accordance with the Declaration of Helsinki. The research received approval from the Research Ethics Committee of The First Affiliated Hospital of Shandong First Medical University and all participants provided written informed consent. Conflicts of interest: The authors declare no competing interests.
Figures




References
-
- Gilhus NE. Myasthenia Gravis. N Engl J Med. 2016;375(26):2570–81. - PubMed
-
- Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30. - PubMed
-
- Yu D, Walker LSK, Liu Z, Linterman MA, Li Z. Targeting T(FH) cells in human diseases and vaccination: rationale and practice. Nat Immunol. 2022;23(8):1157–68. - PubMed
-
- Zhang CJ, Gong Y, Zhu W, Qi Y, Yang CS, Fu Y, Chang G, Li Y, Shi S, Wood K, et al. Augmentation of circulating follicular helper T cells and their impact on autoreactive B cells in myasthenia gravis. J Immunol. 2016;197(7):2610–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

