Effectiveness and safety of cefiderocol treatment in patients with Gram-negative bacterial infections in Spain in the early access programme: results of the PERSEUS study
- PMID: 40131647
- PMCID: PMC12116923
- DOI: 10.1007/s10096-025-05108-6
Effectiveness and safety of cefiderocol treatment in patients with Gram-negative bacterial infections in Spain in the early access programme: results of the PERSEUS study
Abstract
Purpose: We assessed the effectiveness and safety of cefiderocol in patients with Gram-negative bacterial infections, excluding Acinetobacter spp., in the early access programme (EAP) in Spain.
Methods: The retrospective, multicentre PERSEUS study (2018-2022) enrolled hospitalised patients with serious Gram-negative infections, except Acinetobacter spp., who received first-time cefiderocol for ≥ 72 h following requests through the EAP. Clinical cure at end of treatment, all-cause mortality at Day 28, cefiderocol use, and adverse drug reactions (ADRs) were the key outcomes.
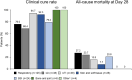
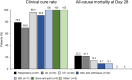
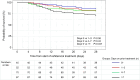
Results: Overall, 261 patients were eligible for analysis. Median (interquartile range) age was 61 (49-68) years, 202 (77.4%) were male and 165 (63.2%) were in the intensive care unit. The most frequent diagnoses were respiratory tract infection (47.9%), intra-abdominal infection (14.6%), and urinary tract infection (14.6%). The median (IQR) duration of cefiderocol treatment was 10 (7-14) days. Overall, the clinical cure rate was 80.5% (210/261) and the 28-day mortality rate was 21.5% (56/261). In patients with Pseudomonas aeruginosa infection (66.7% [n = 174], including 73 [42%] with metallo-β-lactamases), the clinical cure rate was 84.5% (147/174) and the 28-day mortality was 17.2% (30/174). Logistic regression analysis showed that prior antibiotic treatment for > 7 days (OR 0.19, 95% CI 0.05-0.56) and mechanical ventilation (OR 0.32, 95% CI 0.15-0.67) were independent negative predictive factors for clinical cure. ADRs occurred in seven patients, six events resolved, and one was fatal (toxic epidermal necrolysis).
Conclusions: Cefiderocol is a valuable option in the treatment of serious Gram-negative bacterial infections, particularly for those caused by P. aeruginosa.
Clinicaltrials: GOV: NCT05789199 (Registration date: 16 February 2023).
Keywords: Pseudomonas aeruginosa; Carbapenem resistance; Cefiderocol; Early appropriate therapy; Gram-negative; Limited treatment options; Multidrug resistance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The study was approved by the institutional review board of Hospital La Princesa, Madrid, on 3 November 2020 (Royal Decree 957/2020), which served as central reference ethics committee. The study was compliant with all legal and regulatory requirements, the International Conference on Harmonisation Good Clinical Practice E6 guidelines and the Declaration of Helsinki. Consent to participate: According to Spanish regulations, the informed consent for participants was waived by the regulator because patients completed their treatment prior to initiation of this retrospective study and the study represented no harm for the participants. Consent to publication: Not applicable. Competing interests: Julian Torre-Cisneros has received educational grants and fee for advisory activities from Shionogi, Pfizer, MSD, Menarini; and unrestricted research grants from Pfizer and MSD. Benito Almirante received honoraria from Shionogi & Co., Ltd., Osaka, Japan, for participation in this study. Carmen De La Fuente Martos received honoraria from Shionogi & Co., Ltd., Osaka, Japan, for participation in this study. Pedro Rascado has received educational grants and consultancy fees for advisory activities from Pfizer, MSD, Shionogi, and Menarini. Miguel Salavert Lletí has received honoraria for lectures and advisory boards from Angelini, Janssen, Menarini, MSD, Pfizer, Shionogi and Viatris, and educational grants from Gilead and Tedec-Meiji Farma. Miguel Sánchez-García received speaker fees from Shionogi & Co., Spain. Maria Cruz Soriano-Cuesta has received grants for attending medical courses and conferences, and/or received fees for consulting or educational programs from Pfizer, Astellas, MSD, Shionogi, Menarini, Gilead, Mundipharma, and Viatris.Alex Soriano has received honoraria for lectures and advisory boards from Shionogi, Pfizer, Menarini, Angelini, Advance Pharma and Gilead, and grants from Pfizer and Gilead. Ricard Ferrer has received honoraria for lectures from Gilead, Menarini, MSD, Shionogi, and ThermoFisher; consulting fees from Cytosorbent, Inoterm, and Pfizer; and holds stocks or stock options from Grifols. Jessica Sarda, A. Javier Gonzalez Calvo, Stefano Verardi, Andreas Karas are employees of Shionogi.
Figures


References
-
- Centers for Disease Control and Prevention (2022) COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. Atlanta, GA: U.S. Department of Health and Human Services, CDC. Available at: https://www.cdc.gov/drugresistance/covid19.html. 10.15620/cdc:117915
-
- Lodise TP, Berger A, Altincatal A et al (2019) Antimicrobial resistance or delayed appropriate therapy-does one influence outcomes more than the other among patients with serious infections due to carbapenem-resistant versus carbapenem-susceptible Enterobacteriaceae?? Open Forum Infect Dis 6:ofz194. 10.1093/ofid/ofz194 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

