Treatment patterns and outcomes in HER2-low metastatic breast cancer patients previously treated with chemotherapy: a US real-world cohort study
- PMID: 40131662
- PMCID: PMC12006274
- DOI: 10.1007/s10549-025-07649-y
Treatment patterns and outcomes in HER2-low metastatic breast cancer patients previously treated with chemotherapy: a US real-world cohort study
Abstract
Purpose: Real-world outcomes are poorly understood for patients with human epidermal growth factor receptor 2 (HER2)-low (immunohistochemistry 1+ or 2+ with negative in situ hybridization) metastatic breast cancer (mBC).
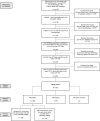
Methods: Using for the first time a nationwide electronic health record-derived de-identified database, we assessed demographics, treatment patterns, and outcomes of patients with HER2-low mBC who previously received one line of chemotherapy in the metastatic setting. The post-chemotherapy line was termed the index line of therapy (LOT).
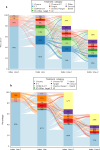
Results: 3765 patients [hormone receptor (HR)-positive: 78.8%, HR-negative: 21.0%] met the inclusion criteria (1 January 2011-30 April 2023). 61.7% of HR-positive patients received endocrine therapy prior to the index LOT. The largest patient percentage received single-agent chemotherapy at the index and subsequent two LOTs. For the overall cohort, the median real-world time to treatment discontinuation/death was 4.1 months (95% CI: 3.9-4.2) and the median real-world time to next treatment/death was 5.1 months (95% CI: 4.8-5.3) from the index LOT. Median real-world overall survival (all patients) was 15.8 months (95% confidence interval: 15.2-16.5, median follow-up = 54.5 months) from the index LOT.
Conclusion: These data highlight the unmet clinical needs of patients with HER2-low mBC by characterizing the treatment patterns and poor outcomes in this population on the current standard of care.
Keywords: Cohort study; HER2-low; Metastatic breast cancer; Real-world evidence; T-DXd.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: SM: Grants or contracts (to institution): AstraZeneca, Daiichi Sankyo, Duality Bio, Genentech, Nuvation, Seagen; consulting fees: AstraZeneca, Daiichi Sankyo, Duality Bio, Eli Lilly, Genentech, Gilead, GSK, Macrogenics; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: AstraZeneca, Daiichi Sankyo, Seagen; support for attending meetings and/or travel: AstraZeneca, Daiichi Sankyo, Seagen. SZ, YX, SH: Employees of Daiichi Sankyo, Inc. DB, AS: Employees of Daiichi Sankyo Europe GmbH. KD: Employee of Daiichi Sankyo UK Ltd. ZM: Employee of AstraZeneca. WJ: Grants or contracts: AstraZeneca, Daiichi Sankyo; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: AstraZeneca, BMS, Daiichi Sankyo, Eisai, Lilly (France), MSD, Novartis, Pfizer, Roche, Seagen; support for attending meetings and/or travel: AstraZeneca, Chugai, Eisai, GSK, Lilly (France), Novartis, Pfizer, Pierre Fabre, Roche, Sanofi Aventis; participation on a Data Safety Monitoring Board or Advisory Board: AstraZeneca, BMS, Daiichi Sankyo, Eisai, Gilead, Lilly (France), MSD, Novartis, Pfizer, Roche, Seagen. Ethical approval: All the results presented in this article are in aggregate form, and no personally identifiable information was used for this study. The data were de-identified and subject to obligations to prevent re-identification and protect patient confidentiality.
Figures

References
-
- Tarantino P et al (2020) HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 38:1951–1962. 10.1200/jco.19.02488 - PubMed
-
- Hamilton EP et al (2024) Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): updated survival results of DESTINY-Breast03. J Clin Oncol 42:1025–1025. 10.1200/JCO.2024.42.16_suppl.1025
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

