Parallel patterns of age-related working memory impairment in marmosets and macaques
- PMID: 40131878
- PMCID: PMC11984434
- DOI: 10.18632/aging.206225
Parallel patterns of age-related working memory impairment in marmosets and macaques
Abstract
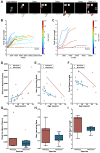
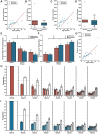
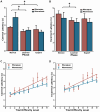
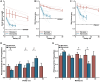
As humans age, some experience cognitive impairment while others do not. When impairment does occur, it is not expressed uniformly across cognitive domains and varies in severity across individuals. Translationally relevant model systems are critical for understanding the neurobiological drivers of this variability, which is essential to uncovering the mechanisms underlying the brain's susceptibility to the effects of aging. As such, non-human primates (NHPs) are particularly important due to shared behavioral, neuroanatomical, and age-related neuropathological features with humans. For many decades, macaque monkeys have served as the primary NHP model for studying the neurobiology of cognitive aging. More recently, the common marmoset has emerged as an advantageous model for this work due to its short lifespan that facilitates longitudinal studies. Despite their growing popularity as a model, whether marmosets exhibit patterns of age-related cognitive impairment comparable to those observed in macaques and humans remains unexplored. To address this major limitation for the development and evaluation of the marmoset as a model of cognitive aging, we directly compared working memory ability as a function of age in macaques and marmosets on the identical task. We also implemented varying delays to further tax working memory capacity. Our findings demonstrate that marmosets and macaques exhibit remarkably similar age-related working memory deficits, with macaques performing better than marmosets on longer delays. These results highlight the similarities and differences between the two most commonly used NHP models and support the value of the marmoset as a model for cognitive aging research within the neuroscience community.
Keywords: aging; animal model; cognition; comparative cognition; monkey.
Conflict of interest statement
Figures
Update of
-
Parallel patterns of cognitive aging in marmosets and macaques.bioRxiv [Preprint]. 2024 Jul 23:2024.07.22.604411. doi: 10.1101/2024.07.22.604411. bioRxiv. 2024. Update in: Aging (Albany NY). 2025 Mar 24;17(3):778-797. doi: 10.18632/aging.206225. PMID: 39091859 Free PMC article. Updated. Preprint.
References
-
- Cook Maher A, Makowski-Woidan B, Kuang A, Zhang H, Weintraub S, Mesulam MM, Rogalski E. Neuropsychological Profiles of Older Adults with Superior versus Average Episodic Memory: The Northwestern "SuperAger" Cohort. J Int Neuropsychol Soc. 2022; 28:563–73. 10.1017/S1355617721000837 - DOI - PMC - PubMed
-
- Vanderlip CR, Stark CEL, Initiative ADN. Digital cognitive assessments as low-burden markers for predicting future cognitive decline and tau accumulation across the Alzheimer’s spectrum. bioRxiv. 2024; 2024.05.23.595638. Available from: https://www.biorxiv.org/content/10.1101/2024.05.23.595638v1. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

