Time-restricted eating in early-stage Huntington's disease: A 12-week interventional clinical trial protocol
- PMID: 40131943
- PMCID: PMC11936236
- DOI: 10.1371/journal.pone.0319253
Time-restricted eating in early-stage Huntington's disease: A 12-week interventional clinical trial protocol
Abstract
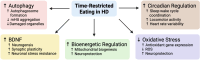
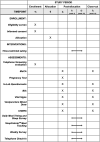
Huntington's disease (HD) is a devastating neurodegenerative disorder characterized by a variety of debilitating symptoms including abnormal motor control, cognitive impairment, and psychiatric disturbances. Despite significant efforts, efficacious treatments to alter the course of HD remain elusive, highlighting the need to explore new therapeutic strategies, including lifestyle changes that may delay the onset of symptoms and slow disease progression. Recent research indicates that time-restricted eating (TRE), a type of intermittent fasting where caloric intake is confined to a specific time window each day, may be beneficial in treating neurodegenerative diseases like HD. TRE has been found to enhance mitochondrial function, stimulate autophagy, lower oxidative stress, and improve cognitive performance. Although TRE has shown potential in HD animal models and non-HD populations, it has yet to be analyzed for safety, feasibility, and efficacy in persons with HD. Therefore, we propose a prospective interventional, open-label, single-arm, pilot study of 25 participants with late prodromal and early manifest HD to evaluate participant adherence to TRE diet - specifically, maintaining a 6-8-hour eating window every day for 12 weeks. Secondary measures will include pre- versus post-intervention assessment of body composition via bioelectrical impedance analysis, vital signs and safety labs, serum biomarkers of neurodegeneration, and standard HD behavioral, cognitive, and motor function clinical scales. Additional exploratory measures will evaluate sleep quality, physical activity, mood, dietary composition, and mitochondrial function. We expect that the diet will be safe, feasible, and may also improve biomarkers of disease progression in persons with HD. We anticipate this study will lay the foundation for future large-scale clinical trials to further evaluate the clinical efficacy of TRE in HD. This study has been registered on July 8, 2024 with ClinicalTrials.gov registration number NCT06490367 (https://clinicaltrials.gov/study/NCT06490367).
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

